Members Login

Channels
Special Offers & Promotions
Products
Contact
All articles from
Agar announces the Kleindiek Nanotechnik positioning tools are now available for use with benchtop SEM systems
Feb 16, 2013
Medicyte Starts its Involvement in the Prestigious Hepatic Microfluidic Bioreactor Project
May 3, 2011
Copley Scientific delivers eight further dissolution testers to thriving Melbourn Scientific
Mar 10, 2011
QIAGEN launches ScreenGel software for QIAxcel multicapillary gel electrophoresis systems
Feb 25, 2011
Thermo Fisher Scientific Introduces Centrifuge for Large-volume Blood Banking and Bioprocessing
Nov 16, 2010
Dionex Demonstrates Simultaneous Determination of Anions and Cations in Saline and Drinking Water Matrices
Nov 5, 2010
New Lab901 Technical Note: The ScreenTape Degradation Value (SDV), a new standard for RNA QC
Oct 28, 2010
Peptide Synthesis Webinar Series Introduces Second Installment: Synthesis of Difficult Peptide Sequences
Oct 26, 2010
Thermo Fisher Scientific Announces Reliable Method for Rapid and Accurate Analysis of Trace Elements in Honey
Oct 22, 2010
Stratophase Presents Biothreat Detection Poster at SPIE Security and Defence Conference
Sep 17, 2010
Thermo Fisher Scientific Announces Enhancements to Its Quantitative and Qualitative Proteomics Tools at ASMS 2010
May 26, 2010
Dionex Releases Protein and Antibody Therapeutics and Nucleic Acid Therapeutics Application Notebooks
May 20, 2010
Dionex Demonstrates Two Dimensional Ion Chromatography for the Determination of Total Phosphorus
Apr 28, 2010
BioFocus DPI and Oncodesign forge alliance to provide integrated oncology drug discovery services
Oct 6, 2009
New Hardware/Software Workflow Helps Food and Environmental Laboratories Meet Tough Monitoring Requirements
Aug 18, 2009
Media Partners


 EIBF, the UK registered charity that promotes business education for engineers and scientists, and the National Physical Laboratory (NPL), the UK’s National Metrology Institute, are partnering on an MBA scholarship scheme that will provide business education for scientists. The collaboration will provide MBA scholarships worth £50,000 to eight UK science graduates each year...
EIBF, the UK registered charity that promotes business education for engineers and scientists, and the National Physical Laboratory (NPL), the UK’s National Metrology Institute, are partnering on an MBA scholarship scheme that will provide business education for scientists. The collaboration will provide MBA scholarships worth £50,000 to eight UK science graduates each year...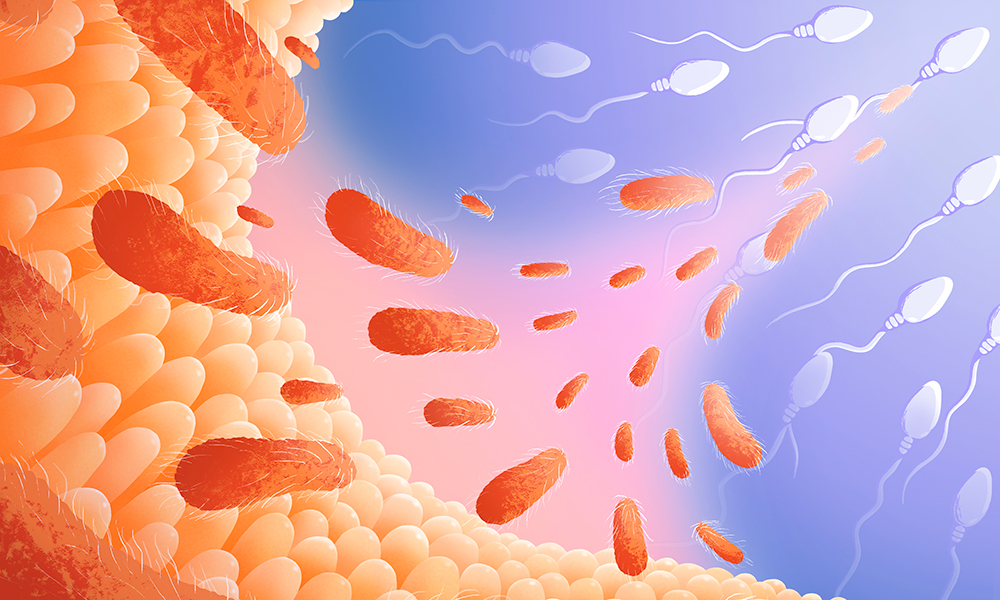 A study from the Hackett group at EMBL Rome shows that disrupting the gut microbiome of male mice increases the risk of disease in their future offspring. Researchers from the Hackett group at the European Molecular Biology Laboratory (EMBL) in Rome in collaboration with the Bork and the Zimmermann groups at EMBL Heidelberg changed the composition of the gut microbiota in male mice through common antibiotics, inducing a condition called dysbiosis, and found that...
A study from the Hackett group at EMBL Rome shows that disrupting the gut microbiome of male mice increases the risk of disease in their future offspring. Researchers from the Hackett group at the European Molecular Biology Laboratory (EMBL) in Rome in collaboration with the Bork and the Zimmermann groups at EMBL Heidelberg changed the composition of the gut microbiota in male mice through common antibiotics, inducing a condition called dysbiosis, and found that...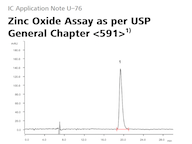 USP has updated the General Chapter <591> «Zinc Determination» monograph to include ion chromatography as the preferred method for measuring zinc oxide. USP 41–NF 36 was developed in partnership with Metrohm and replaces manual titration methods. The new method is approved by the FDA and benefits users with more ease of use and reliability. Zinc oxide is used in various skin care creams, drugs, and drug products....
USP has updated the General Chapter <591> «Zinc Determination» monograph to include ion chromatography as the preferred method for measuring zinc oxide. USP 41–NF 36 was developed in partnership with Metrohm and replaces manual titration methods. The new method is approved by the FDA and benefits users with more ease of use and reliability. Zinc oxide is used in various skin care creams, drugs, and drug products.... From August 28 through 30, 2019, Metrohm will be hosting the 3rd Global User Meeting IC in Herisau, Switzerland. The focus will be on latest developments in ion chromatography, which will be presented by our application specialists as well as guest speakers from various industries and academia....
From August 28 through 30, 2019, Metrohm will be hosting the 3rd Global User Meeting IC in Herisau, Switzerland. The focus will be on latest developments in ion chromatography, which will be presented by our application specialists as well as guest speakers from various industries and academia.... The glass free electrode makes it particularly safe to use in food production as no glass splinters can get into the food if the electrode breaks. Unlike glass pH electrodes, ISFET pH sensors are highly resilient and do not require wet storage. They give reliable results under tough conditions. The PCE-ISFET pH tester’s highly sensitive electronics can achieve a resolution and accuracy of 0.005 pH, at a measurement range of 0.00 ... 16.00 pH.
The glass free electrode makes it particularly safe to use in food production as no glass splinters can get into the food if the electrode breaks. Unlike glass pH electrodes, ISFET pH sensors are highly resilient and do not require wet storage. They give reliable results under tough conditions. The PCE-ISFET pH tester’s highly sensitive electronics can achieve a resolution and accuracy of 0.005 pH, at a measurement range of 0.00 ... 16.00 pH. AMSBIO announces iMatrix recombinant Laminin-511 E8 fragments, an innovative cell culture matrix compatible with a wide variety of cell types, and exceptionally well suited for pluripotent stem cells.
AMSBIO announces iMatrix recombinant Laminin-511 E8 fragments, an innovative cell culture matrix compatible with a wide variety of cell types, and exceptionally well suited for pluripotent stem cells. IDEX Health & Science, LLC has announced the acquisition of thinXXS Microtechnology that will accelerate growth of its microfluidics consumables business.
IDEX Health & Science, LLC has announced the acquisition of thinXXS Microtechnology that will accelerate growth of its microfluidics consumables business. Fast Track Diagnostics S.à.r.l., one of the leading global suppliers of syndromic real-time PCR multiplexing kits, has been partnering for several months with UgenTec NV, an innovative European medical device software company that develops artificially intelligent PCR interpretation software.
Fast Track Diagnostics S.à.r.l., one of the leading global suppliers of syndromic real-time PCR multiplexing kits, has been partnering for several months with UgenTec NV, an innovative European medical device software company that develops artificially intelligent PCR interpretation software. I-Stem was formed in 2005 through a collaboration between Inserm, the French National Institute of Health and Medical Research, and AFM-Telethon the French Association against Myopathies. I-Stem is the largest French laboratory for research and development dedicated to human pluripotent stem cells....
I-Stem was formed in 2005 through a collaboration between Inserm, the French National Institute of Health and Medical Research, and AFM-Telethon the French Association against Myopathies. I-Stem is the largest French laboratory for research and development dedicated to human pluripotent stem cells.... By targeted filtration of patient serum using a specific binding method removes protein contaminant to isolate exosome at 95% high purity. Using this proprietary filtration method, the purified tumor exosome does not contain any polymer or detergent (SDS, NP-40 or Triton). The purified tumor exosome can be used for RNA or protein analysis techniques such as qRT-PCR, microarray and mass spectrometry....
By targeted filtration of patient serum using a specific binding method removes protein contaminant to isolate exosome at 95% high purity. Using this proprietary filtration method, the purified tumor exosome does not contain any polymer or detergent (SDS, NP-40 or Triton). The purified tumor exosome can be used for RNA or protein analysis techniques such as qRT-PCR, microarray and mass spectrometry.... Immune evasion is one of the identifying hallmarks of cancer, and researchers worldwide are trying to identify the complex mechanisms that enable cancer cells to evade the host’s immune system. Cancer cells use the indoleamine 2,3-dioxygenase (IDO) pathway to suppress the host’s immune response in order to facilitate survival, growth, invasion, and metastasis of malignant cells....
Immune evasion is one of the identifying hallmarks of cancer, and researchers worldwide are trying to identify the complex mechanisms that enable cancer cells to evade the host’s immune system. Cancer cells use the indoleamine 2,3-dioxygenase (IDO) pathway to suppress the host’s immune response in order to facilitate survival, growth, invasion, and metastasis of malignant cells.... Circulating cfDNA is normally comprised of DNA fragments from healthy cells. However, apoptotic cells, necrotic cells, cancer cells, and intact cells all release cfDNA into the bloodstream. In addition, fetal cfDNA can be isolated from peripheral maternal blood. The utility and value of using cfDNA from serum and plasma for non-invasive molecular diagnostics...
Circulating cfDNA is normally comprised of DNA fragments from healthy cells. However, apoptotic cells, necrotic cells, cancer cells, and intact cells all release cfDNA into the bloodstream. In addition, fetal cfDNA can be isolated from peripheral maternal blood. The utility and value of using cfDNA from serum and plasma for non-invasive molecular diagnostics... This laboratory mixer is designed to provide variable speed liquid mixing in a compact, durable and user-friendly unit. And the rotary shaker's compact footprint will fit into most incubators allowing for controlled environment mixing....
This laboratory mixer is designed to provide variable speed liquid mixing in a compact, durable and user-friendly unit. And the rotary shaker's compact footprint will fit into most incubators allowing for controlled environment mixing.... With an Omega Lum G Gel Documentation system that is no longer the case.
With an Omega Lum G Gel Documentation system that is no longer the case. At the push of a button, VOYAGER II pipettes allow you to expand tip spacing anywhere between 4.5 mm and 33 mm (depending on the model). This enables rapid and optimised multichannel pipetting between microplates, tube racks and gel boxes of different sizes and formats. The VOYAGER II pipette incorporates a new 135-degree rotatable body enabling users to always hold the pipette in a comfortable working position. Additionally, the reduced...
At the push of a button, VOYAGER II pipettes allow you to expand tip spacing anywhere between 4.5 mm and 33 mm (depending on the model). This enables rapid and optimised multichannel pipetting between microplates, tube racks and gel boxes of different sizes and formats. The VOYAGER II pipette incorporates a new 135-degree rotatable body enabling users to always hold the pipette in a comfortable working position. Additionally, the reduced...
 Herolab, the German manufacturer of equipment for life science, have upgraded the ChemoLum multi-imaging system which guarantees excellent results for chemiluminescence as well as fluorescence applications. The major advance has been the introduction of a new high quality f0.95 lens which has enabled the overall size of the system to be reduced....
Herolab, the German manufacturer of equipment for life science, have upgraded the ChemoLum multi-imaging system which guarantees excellent results for chemiluminescence as well as fluorescence applications. The major advance has been the introduction of a new high quality f0.95 lens which has enabled the overall size of the system to be reduced....
 The Countstar Fluorescence Cell Analyser offers a number of key advantages over existing technologies. It has some significant differences from other cell analysis devices and out-performs many others on the market.The combination of bright-field illumination with up to 5X magnification, 4 fluorescence excitation wavelengths and 5 emission wavelength filters give the system a very wide and flexible choice of sample options....
The Countstar Fluorescence Cell Analyser offers a number of key advantages over existing technologies. It has some significant differences from other cell analysis devices and out-performs many others on the market.The combination of bright-field illumination with up to 5X magnification, 4 fluorescence excitation wavelengths and 5 emission wavelength filters give the system a very wide and flexible choice of sample options.... Herolab GmbH Laborgeräte of Wiesloch in Germany manufactures products for Life Science research, diagnostics and production. For the past 36 years the Company has produced a range of exciting products. Alongside the well-known centrifuge and gel documentation ranges is also the UV products division. The UV products division has a number of key products that are used in life science and general laboratories as well as for industrial applications....
Herolab GmbH Laborgeräte of Wiesloch in Germany manufactures products for Life Science research, diagnostics and production. For the past 36 years the Company has produced a range of exciting products. Alongside the well-known centrifuge and gel documentation ranges is also the UV products division. The UV products division has a number of key products that are used in life science and general laboratories as well as for industrial applications.... Vivantis is pleased to announce the new 2X At Taq Master Mix (Hot Start). For a limited time there is a 20% off introductory offer. This more powerful tool will now give you more promising results and yield in your DNA amplification work. The 2X At Taq Master Mix is an optimised ready-to-use 2X concentrated DNA amplification mixture containing At Taq DNA Polymerase, reaction buffer, dNTPs, and MgCl2. At Taq DNA polymerase is a complex of specific anti-Taq monoclonal antibody with...
Vivantis is pleased to announce the new 2X At Taq Master Mix (Hot Start). For a limited time there is a 20% off introductory offer. This more powerful tool will now give you more promising results and yield in your DNA amplification work. The 2X At Taq Master Mix is an optimised ready-to-use 2X concentrated DNA amplification mixture containing At Taq DNA Polymerase, reaction buffer, dNTPs, and MgCl2. At Taq DNA polymerase is a complex of specific anti-Taq monoclonal antibody with... The company has set its goal to provide innovative, high quality products with an efficient, friendly service. The aim of Gel Company is to offer all its products at competitive prices. The focus is to develop, manufacture and supply innovative tools for proteomics, genomics, cell biology, liquid handling and microarray applications to scientists around the world.
The company has set its goal to provide innovative, high quality products with an efficient, friendly service. The aim of Gel Company is to offer all its products at competitive prices. The focus is to develop, manufacture and supply innovative tools for proteomics, genomics, cell biology, liquid handling and microarray applications to scientists around the world.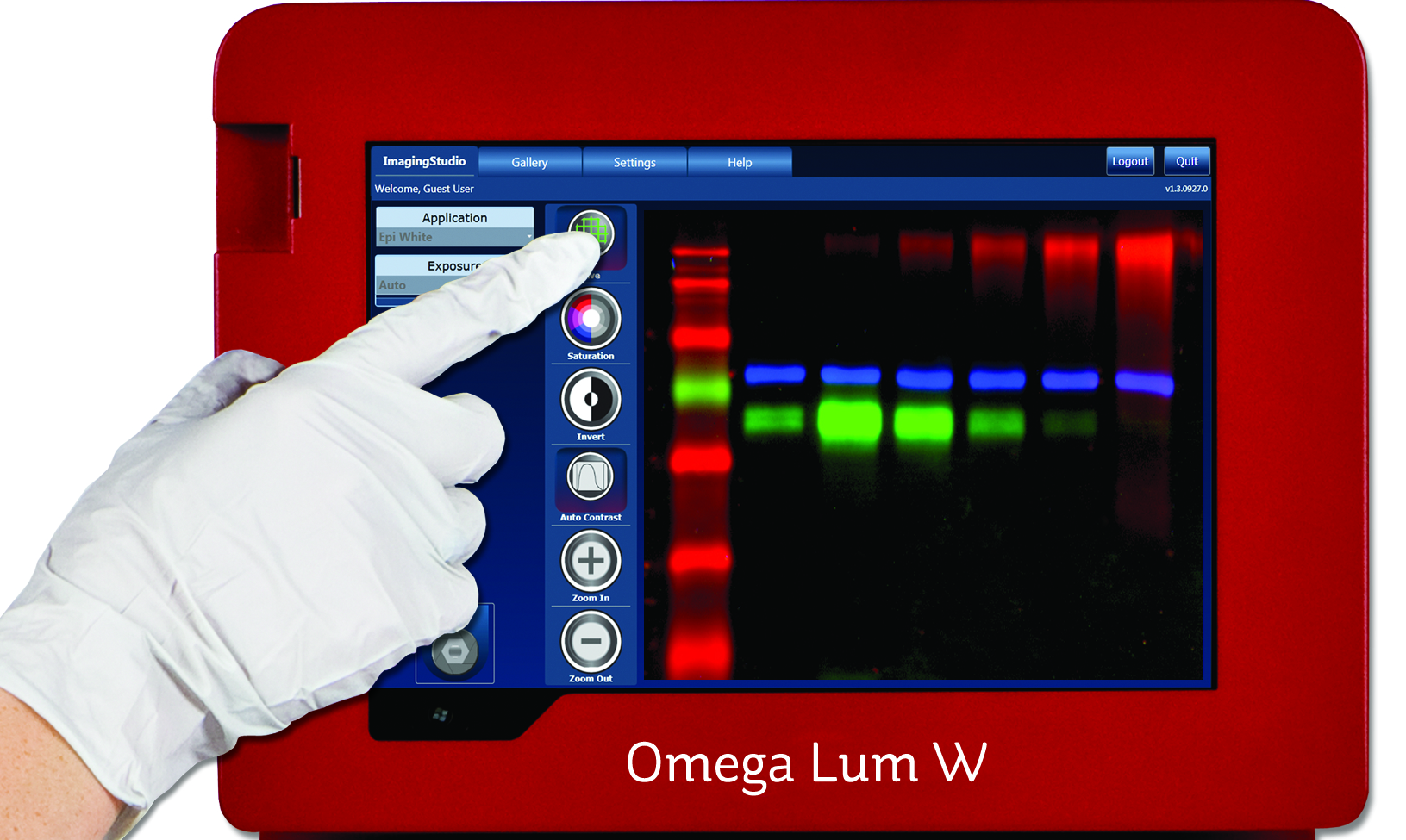
 The HeroDoc Plus from Herolab is a new addition to the gel documentation range of products on offer from the company. This time Herolab have focussed on the entry level end of the market with a compact system for the imaging for gels and blots. Pricing is always important at this end of the market and so Herolab have cleverly configured the product to fit within the limits of most typical budgets....
The HeroDoc Plus from Herolab is a new addition to the gel documentation range of products on offer from the company. This time Herolab have focussed on the entry level end of the market with a compact system for the imaging for gels and blots. Pricing is always important at this end of the market and so Herolab have cleverly configured the product to fit within the limits of most typical budgets....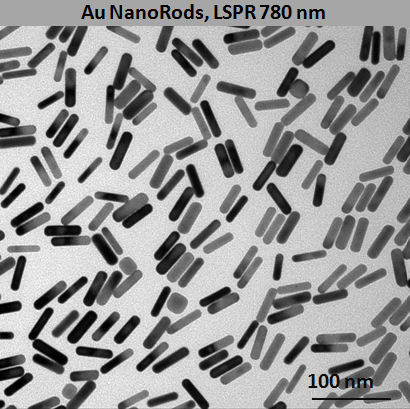
 Research work at the Institut Curie requires petri dishes for a wide range of applications including growing bacteria, yeasts and moulds, making it necessary to regularly produce 10 to 12 different types of media in varying quantities (from one to several litres). The assurance of reliable, high throughput production of these petri dishes, free of any contamination, is critical to ongoing research work. Fatima Dekmous, Departmental Head of the Wash facility at Institut Curie commented...
Research work at the Institut Curie requires petri dishes for a wide range of applications including growing bacteria, yeasts and moulds, making it necessary to regularly produce 10 to 12 different types of media in varying quantities (from one to several litres). The assurance of reliable, high throughput production of these petri dishes, free of any contamination, is critical to ongoing research work. Fatima Dekmous, Departmental Head of the Wash facility at Institut Curie commented... Leveraging an existing knowledgeable local sales and service team – INTEGRA Biosciences Deutschland GmbH was set up to market and provide top quality local support for the company’s innovative laboratory tools for liquid handling, media preparation, sterilisation and cell cultivation. Jan Müller, General Manager of INTEGRA Biosciences Deutschland GmbH, commented: “We are very happy to announce the opening of this...
Leveraging an existing knowledgeable local sales and service team – INTEGRA Biosciences Deutschland GmbH was set up to market and provide top quality local support for the company’s innovative laboratory tools for liquid handling, media preparation, sterilisation and cell cultivation. Jan Müller, General Manager of INTEGRA Biosciences Deutschland GmbH, commented: “We are very happy to announce the opening of this... The Filtration Society 50th Anniversary Conference will take place on 13-14 November 2014 at the Riverside Innovation Centre, Chester, UK. With a confirmed line up of international speakers drawn from industry and academia, as well as a well-supported trade exhibition, the organisers are expecting to welcome conference participants from around the world. The Filtration Society was formed in London in 1964 and has the objectives of...
The Filtration Society 50th Anniversary Conference will take place on 13-14 November 2014 at the Riverside Innovation Centre, Chester, UK. With a confirmed line up of international speakers drawn from industry and academia, as well as a well-supported trade exhibition, the organisers are expecting to welcome conference participants from around the world. The Filtration Society was formed in London in 1964 and has the objectives of... The chemistry specialist, which excels in pre-clinical development projects, has decided to adopt a more novel approach to manufacturing API in a bid to overcome the often time-consuming and costly process of batch manufacturing. Denise Bowser, commercial director at Onyx Scientific, said: “The move to continuous flow manufacturing has been seen by a handful of companies in the sector to date so we feel that we are very much at the forefront of technological advances when it comes to...
The chemistry specialist, which excels in pre-clinical development projects, has decided to adopt a more novel approach to manufacturing API in a bid to overcome the often time-consuming and costly process of batch manufacturing. Denise Bowser, commercial director at Onyx Scientific, said: “The move to continuous flow manufacturing has been seen by a handful of companies in the sector to date so we feel that we are very much at the forefront of technological advances when it comes to... Developed to give improved growth rates of Listeria over traditional selective enrichment media, LEE Broth enhances the expression of target antigens for most commercially available immunological test methods whilst maintaining suppression of non-target organisms.
Developed to give improved growth rates of Listeria over traditional selective enrichment media, LEE Broth enhances the expression of target antigens for most commercially available immunological test methods whilst maintaining suppression of non-target organisms.
 Agar Scientific have been serving microscopists with the supply of accessories and consumables for more than forty years. Together with Kleindiek Nanotechnik, Agar supplies a range of micro-manipulation, nano-positioning and probing systems for users in academic research and industry. The latest development is the introduction of Kleindiek's micromanipulators and prober shuttles for use with benchtop SEMs...
Agar Scientific have been serving microscopists with the supply of accessories and consumables for more than forty years. Together with Kleindiek Nanotechnik, Agar supplies a range of micro-manipulation, nano-positioning and probing systems for users in academic research and industry. The latest development is the introduction of Kleindiek's micromanipulators and prober shuttles for use with benchtop SEMs... Bibby Scientific is the home of four famous brands - Jenway, Stuart, Techne and Electrothermal, all with reputations for product quality and exceptional performance. At Achema 2012 Bibby Scientific will be showcasing a multitude of new products. The Jenway spotlight will be focussed on the new Genova Plus - a spectrophotometer dedicated to life science analysis, the new Genova Nano - Jenway's first ever micro-volume spectrophotometer and the 6850 - a variable spectral bandwidth double beam spectrophotometer...
Bibby Scientific is the home of four famous brands - Jenway, Stuart, Techne and Electrothermal, all with reputations for product quality and exceptional performance. At Achema 2012 Bibby Scientific will be showcasing a multitude of new products. The Jenway spotlight will be focussed on the new Genova Plus - a spectrophotometer dedicated to life science analysis, the new Genova Nano - Jenway's first ever micro-volume spectrophotometer and the 6850 - a variable spectral bandwidth double beam spectrophotometer... Lab M is adding to its range of CaptivateTM antibody coated paramagnetic particles for the specific immunomagnetic separation (IMS) of micro-organisms. The range currently encompasses products for E. coli O103, E. coli O111, E. coli O145, E. coli O157:H7, E. coli O26, and common serotypes of Salmonella. It is being extended to also include E. coli strains O121, O45, O91. This extended range will cover all ‘The Big Six' strains of non-O157 Shiga-toxin producing E. coli (STECs) now highlighted by the United States Department of Agriculture (USDA) as unacceptable in raw beef products E.coli strains O26, O103, O45, O111, O121 and O145...
Lab M is adding to its range of CaptivateTM antibody coated paramagnetic particles for the specific immunomagnetic separation (IMS) of micro-organisms. The range currently encompasses products for E. coli O103, E. coli O111, E. coli O145, E. coli O157:H7, E. coli O26, and common serotypes of Salmonella. It is being extended to also include E. coli strains O121, O45, O91. This extended range will cover all ‘The Big Six' strains of non-O157 Shiga-toxin producing E. coli (STECs) now highlighted by the United States Department of Agriculture (USDA) as unacceptable in raw beef products E.coli strains O26, O103, O45, O111, O121 and O145... Rigaku Raman Technologies is pleased to announce the release of the world's first dual wavelength handheld Raman analyzer at Pittcon 2012. Built on the stabilized platform of the Xantus-1, our Xantus-2 combines the technology of our 785nm and our 1064nm Raman spectrometers giving you two instruments in one...
Rigaku Raman Technologies is pleased to announce the release of the world's first dual wavelength handheld Raman analyzer at Pittcon 2012. Built on the stabilized platform of the Xantus-1, our Xantus-2 combines the technology of our 785nm and our 1064nm Raman spectrometers giving you two instruments in one... A new University of Aberdeen degree is set to deliver a fresh breed of scientists to address the links between diet and diseases including cancer, diabetes and cardiovascular disease. Nutri-genomics is an emerging area of scientific research which looks at the relationship between what we eat, disease and our own individual genetic make-up...
A new University of Aberdeen degree is set to deliver a fresh breed of scientists to address the links between diet and diseases including cancer, diabetes and cardiovascular disease. Nutri-genomics is an emerging area of scientific research which looks at the relationship between what we eat, disease and our own individual genetic make-up... Verna McErlane, Director of Commercial Operations for miRNA specialist Sistemic, is attending AUSBiotech 2011 in Adelaide, South Australia, 16-19 October. At this event she presented a talk entitled ‘The Challenges of Advancing Stem Cell Therapies into the clinic; a Characterisation Perspective'. The presentation formed part of the ‘Human Health - Clever cell culture for the innovation of biologics' session on 18 October...
Verna McErlane, Director of Commercial Operations for miRNA specialist Sistemic, is attending AUSBiotech 2011 in Adelaide, South Australia, 16-19 October. At this event she presented a talk entitled ‘The Challenges of Advancing Stem Cell Therapies into the clinic; a Characterisation Perspective'. The presentation formed part of the ‘Human Health - Clever cell culture for the innovation of biologics' session on 18 October... Vacuum Expo provides a sizeable and comprehensive exhibition that involves industry leaders presenting new ideas. Delivered with a passion for vacuum technology, Vacuum Expo's remit is to provide a meeting place for users of vacuum technologies and to be a venue for education and training in the use and measurement of vacuum in general industry, in science and manufacturing. It embraces industry needs and challenges for vacuum coating in emerging sectors such as in photovoltaics and LED manufacture and in optical thin films...
Vacuum Expo provides a sizeable and comprehensive exhibition that involves industry leaders presenting new ideas. Delivered with a passion for vacuum technology, Vacuum Expo's remit is to provide a meeting place for users of vacuum technologies and to be a venue for education and training in the use and measurement of vacuum in general industry, in science and manufacturing. It embraces industry needs and challenges for vacuum coating in emerging sectors such as in photovoltaics and LED manufacture and in optical thin films... A new video from Avid Nano demonstrates how easy making precise DLS measurements has become using their W130i dynamic light scattering system with patent pending 5µl BladeCellTM disposable cuvettes...
A new video from Avid Nano demonstrates how easy making precise DLS measurements has become using their W130i dynamic light scattering system with patent pending 5µl BladeCellTM disposable cuvettes... Drs Kevin Mattison*, Ulf Nobbmann and Jason Sanchez of Malvern Instruments will lead a workshop on ‘Characterization techniques for protein therapeutics' - at the 7th Annual PEGS - Protein & Antibody Engineering Summit being held in Boston MA, from 9-13 May 2011...
Drs Kevin Mattison*, Ulf Nobbmann and Jason Sanchez of Malvern Instruments will lead a workshop on ‘Characterization techniques for protein therapeutics' - at the 7th Annual PEGS - Protein & Antibody Engineering Summit being held in Boston MA, from 9-13 May 2011... Genevac Ltd (Ipswich, UK), the solvent evaporation business of SP Industries Inc (Warminster, PA) has entered into an OEM agreement with ion chromatography (IC) specialist Dionex Corp (Sunnyvale, CA), which sees the Genevac RocketTM high speed evaporator being sold as part of Dionex accelerated solvent extraction (ASE®) instruments...
Genevac Ltd (Ipswich, UK), the solvent evaporation business of SP Industries Inc (Warminster, PA) has entered into an OEM agreement with ion chromatography (IC) specialist Dionex Corp (Sunnyvale, CA), which sees the Genevac RocketTM high speed evaporator being sold as part of Dionex accelerated solvent extraction (ASE®) instruments... SIRS-Lab, a molecular diagnostics company based in Jena, Germany, announced the inclusion of the first patient in a 1000-patient clinical study evaluating their molecular pathogen test VYOO. The multicenter trial, performed in 11 key centers across Germany and initiated in February 2011, will deliver data about the utility of the PCR test compared to conventional methods to identify microbes in ICU patients with suspected sepsis...
SIRS-Lab, a molecular diagnostics company based in Jena, Germany, announced the inclusion of the first patient in a 1000-patient clinical study evaluating their molecular pathogen test VYOO. The multicenter trial, performed in 11 key centers across Germany and initiated in February 2011, will deliver data about the utility of the PCR test compared to conventional methods to identify microbes in ICU patients with suspected sepsis... Thermo Fisher Scientific Inc., the world leader in serving science,has announced the new Thermo Scientific ARL PERFORM'X X-ray fluorescence (XRF) spectrometer for advanced materials characterization. The ARL PERFORM'X integrates bulk elemental analysis capabilities with mapping and small spot analysis to create a solution that offers unmatched versatility and performance for the analysis of any solid or liquid sample...
Thermo Fisher Scientific Inc., the world leader in serving science,has announced the new Thermo Scientific ARL PERFORM'X X-ray fluorescence (XRF) spectrometer for advanced materials characterization. The ARL PERFORM'X integrates bulk elemental analysis capabilities with mapping and small spot analysis to create a solution that offers unmatched versatility and performance for the analysis of any solid or liquid sample... Following recent joint development, Malvern Instruments Insitec on-line particle size analyzer will be exhibited on the Sturtevant Inc. booth at Interphex 2011 (March 29-31, New York). The Insitec will be displayed together with a Sturtevant Mill and Schenck Accurate Feeder as the first time introduction of ARTMiS, a new Automated RealTime Milling System.
Following recent joint development, Malvern Instruments Insitec on-line particle size analyzer will be exhibited on the Sturtevant Inc. booth at Interphex 2011 (March 29-31, New York). The Insitec will be displayed together with a Sturtevant Mill and Schenck Accurate Feeder as the first time introduction of ARTMiS, a new Automated RealTime Milling System.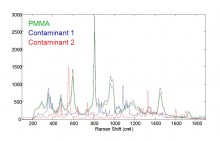 A new application note describing ‘Raman chemical identification of contaminant particles’ is now available on the Malvern Instruments website. It highlights the ability of the company’s Morphologi G3 equipped with Raman microprobe accessory to target particulate impurities, an area of considerable interest to manufacturers of complex, high value products where control of particle size and shape is crucial...
A new application note describing ‘Raman chemical identification of contaminant particles’ is now available on the Malvern Instruments website. It highlights the ability of the company’s Morphologi G3 equipped with Raman microprobe accessory to target particulate impurities, an area of considerable interest to manufacturers of complex, high value products where control of particle size and shape is crucial... Phenomenex Inc., a global leader in the research and manufacture of advanced technologies for the separation sciences, introduces Easy Seal, an advanced seal for GC columns that addresses common problems with installation and reliability. The new accessory is constructed of a proprietary gold-plated alloy that is softer than widely used stainless steel and can be installed without extraordinary pressure...
Phenomenex Inc., a global leader in the research and manufacture of advanced technologies for the separation sciences, introduces Easy Seal, an advanced seal for GC columns that addresses common problems with installation and reliability. The new accessory is constructed of a proprietary gold-plated alloy that is softer than widely used stainless steel and can be installed without extraordinary pressure... Torrey Pines Scientific announces its new EchoTherm Programmable Hot Plates and Digital Stirring Hot Plates for use with large samples in chemical, pharmaceutical, environmental, biochemical, electronic and other laboratories where temperature accuracy, accurate temperature profiles, ease of use, and reproducible sample preparations are a must...
Torrey Pines Scientific announces its new EchoTherm Programmable Hot Plates and Digital Stirring Hot Plates for use with large samples in chemical, pharmaceutical, environmental, biochemical, electronic and other laboratories where temperature accuracy, accurate temperature profiles, ease of use, and reproducible sample preparations are a must... Dr Janine Erler, from The Institute of Cancer Research (ICR), has clinched the pioneer category of the Red's Hot Women Awards, celebrating her significant contribution to the existing body of knowledge on hypoxic tumour cell research. With the help of a hypoxia workstation...
Dr Janine Erler, from The Institute of Cancer Research (ICR), has clinched the pioneer category of the Red's Hot Women Awards, celebrating her significant contribution to the existing body of knowledge on hypoxic tumour cell research. With the help of a hypoxia workstation... Sage-N Research Inc. (Sage-N), the world leader in computational proteomics, have announced a co-marketing agreement with Nonlinear Dynamics who are renowned developers of proteomics software. The collaboration will see Sage-N Research co-market Nonlinear Dynamics' Progenesis LC-MS label-free quantification software alongside its SORCERER proteomics platform. The scientific capabilities of both companies are a natural compliment and the combined offering will facilitate continued progression in the global field of proteomics...
Sage-N Research Inc. (Sage-N), the world leader in computational proteomics, have announced a co-marketing agreement with Nonlinear Dynamics who are renowned developers of proteomics software. The collaboration will see Sage-N Research co-market Nonlinear Dynamics' Progenesis LC-MS label-free quantification software alongside its SORCERER proteomics platform. The scientific capabilities of both companies are a natural compliment and the combined offering will facilitate continued progression in the global field of proteomics... Using Fluidigm Corporation's BioMarkTM System and Digital ArrayTM integrated fluidic circuits (IFCs), researchers from the University of California San Francisco (UCSF) have identified serum microRNAs that can serve as biomarkers for prostate cancer.
Using Fluidigm Corporation's BioMarkTM System and Digital ArrayTM integrated fluidic circuits (IFCs), researchers from the University of California San Francisco (UCSF) have identified serum microRNAs that can serve as biomarkers for prostate cancer. Copley Scientific has delivered a multiple order of eight tablet dissolution testers to Melbourn Scientific as the contract research organisation extends its testing capabilities in response to increasing client demand. The eight DIS6000 systems, one of the most compact dissolution testers available, have been installed and commissioned with full support from the Copley Scientific team who completed installation and operation qualification (IQ/OQ) on site...
Copley Scientific has delivered a multiple order of eight tablet dissolution testers to Melbourn Scientific as the contract research organisation extends its testing capabilities in response to increasing client demand. The eight DIS6000 systems, one of the most compact dissolution testers available, have been installed and commissioned with full support from the Copley Scientific team who completed installation and operation qualification (IQ/OQ) on site... Markes International (Llantrisant, UK) has announced that it will preview a new thermal desorption instrument, the CIA AdvantageTM, at PittCon 2011. The CIA Advantage will be an invaluable tool for analytical chemists who measure VOCs in air samples using canisters, such as US EPA Method TO-15 and other methods involving an extended range of such pollutants, and who want increased work-flow productivity and flexibility. This, coupled with the additional functionality of sorbent tube analysis, will make the CIA Advantage a unique instrument in its field...
Markes International (Llantrisant, UK) has announced that it will preview a new thermal desorption instrument, the CIA AdvantageTM, at PittCon 2011. The CIA Advantage will be an invaluable tool for analytical chemists who measure VOCs in air samples using canisters, such as US EPA Method TO-15 and other methods involving an extended range of such pollutants, and who want increased work-flow productivity and flexibility. This, coupled with the additional functionality of sorbent tube analysis, will make the CIA Advantage a unique instrument in its field... Phenom-World helps you to stay competitive in a world where critical dimensions are continuously getting smaller with the new generation PhenomTM G2 desktop scanning electron microscope (SEM). The Phenom G2 offers direct access to the high-resolution and high-quality imaging necessary in a large variety of applications. It is an affordable solution that enables engineers, technicians, researchers and educational professionals to visualize micron and submicron structures.
Phenom-World helps you to stay competitive in a world where critical dimensions are continuously getting smaller with the new generation PhenomTM G2 desktop scanning electron microscope (SEM). The Phenom G2 offers direct access to the high-resolution and high-quality imaging necessary in a large variety of applications. It is an affordable solution that enables engineers, technicians, researchers and educational professionals to visualize micron and submicron structures. Teem Photonics, France, a world leader in passively Q-switched microchip lasers, has chosen PI's P-611 NanoCube® piezo stage - a high-precision, multi-axis piezo nanopositioning system - for its new µFab3D microfabrication system. Typically used in microfluidics, cell biology and the manufacture of photonic crystal structures in micro-optics, the µFab3D system uses two-photon absorption to produce 3D polymer, protein, metal or other biomaterials microstructures...
Teem Photonics, France, a world leader in passively Q-switched microchip lasers, has chosen PI's P-611 NanoCube® piezo stage - a high-precision, multi-axis piezo nanopositioning system - for its new µFab3D microfabrication system. Typically used in microfluidics, cell biology and the manufacture of photonic crystal structures in micro-optics, the µFab3D system uses two-photon absorption to produce 3D polymer, protein, metal or other biomaterials microstructures... The MERLIN Viscometer is a high performance rotational viscometer capable of both steady shear and yield stress testing in a rugged, compact size footprint. Designed for performing both routine rheological tests such as single point viscosity checks for QC, to complex rheological evaluation for R&D, the MERLIN Viscometer is ideal for investigating the mixing, stirring, and process flow characteristics of fluid systems...
The MERLIN Viscometer is a high performance rotational viscometer capable of both steady shear and yield stress testing in a rugged, compact size footprint. Designed for performing both routine rheological tests such as single point viscosity checks for QC, to complex rheological evaluation for R&D, the MERLIN Viscometer is ideal for investigating the mixing, stirring, and process flow characteristics of fluid systems... Benefiting from an all polypropylene cartridge construction - ChemifilTM cartridges from Porvair Filtration Group offer an ideal solution for the filtration of aggressive chemicals including acids, alkalis, solvents and etchants.
Benefiting from an all polypropylene cartridge construction - ChemifilTM cartridges from Porvair Filtration Group offer an ideal solution for the filtration of aggressive chemicals including acids, alkalis, solvents and etchants. QIAGEN launches ScreenGel software for convenient DNA fragment and RNA analysis with the QIAxcel® multicapillary gel electrophoresis systems. The software combines the highest possible flexibility for researchers with the ability for standardized and reproducible operations in routine labs.
QIAGEN launches ScreenGel software for convenient DNA fragment and RNA analysis with the QIAxcel® multicapillary gel electrophoresis systems. The software combines the highest possible flexibility for researchers with the ability for standardized and reproducible operations in routine labs. Oxford Gene Technology (OGT), provider of innovative clinical genetics and diagnostic solutions to advance molecular medicine, has expanded its CytoSureTM product range to include high-throughput genomic DNA labelling kits and sample tracking spike-ins. These new products will further streamline workflow and minimise sample tracking errors when performing array comparative genomic hybridisation (aCGH).
Oxford Gene Technology (OGT), provider of innovative clinical genetics and diagnostic solutions to advance molecular medicine, has expanded its CytoSureTM product range to include high-throughput genomic DNA labelling kits and sample tracking spike-ins. These new products will further streamline workflow and minimise sample tracking errors when performing array comparative genomic hybridisation (aCGH). Agilent Automation Solutions has announced a new white paper entitled 'An introduction to High-throughput Microchromatography' which introduces a highly versatile quantitative sample preparation technique for protein analysis.
Agilent Automation Solutions has announced a new white paper entitled 'An introduction to High-throughput Microchromatography' which introduces a highly versatile quantitative sample preparation technique for protein analysis. Dionex is proud to announce a new high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD)-based method for the determination of hydroxymethylfurfural (HMF) in samples ranging from food (honey and pancake syrup) to treated biomass (corn stover and wood hydrolysate). Application Note 270: Determination of Hydroxymethylfurfural in Honey and Biomass demonstrates that this method has a broad linear range, high precisions, and low detection limits. This system configuration requires only addition of deionized water for continuous operation
Dionex is proud to announce a new high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD)-based method for the determination of hydroxymethylfurfural (HMF) in samples ranging from food (honey and pancake syrup) to treated biomass (corn stover and wood hydrolysate). Application Note 270: Determination of Hydroxymethylfurfural in Honey and Biomass demonstrates that this method has a broad linear range, high precisions, and low detection limits. This system configuration requires only addition of deionized water for continuous operation Interchangeable technology from Flexicon Liquid Filling, a business division of Watson-Marlow Pumps Group, has helped family-run diagnostic reagent manufacturer, Hart Biologicals Ltd, accommodate business growth with the recent upgrade to an FF30 tabletop bottle filling and capping machine.
Interchangeable technology from Flexicon Liquid Filling, a business division of Watson-Marlow Pumps Group, has helped family-run diagnostic reagent manufacturer, Hart Biologicals Ltd, accommodate business growth with the recent upgrade to an FF30 tabletop bottle filling and capping machine. Seward Ltd, manufacturer and developer of the world leading, patented Stomacher® Laboratory Paddle Blender range for over 40 years, has moved to new premises within Worthing, UK. The new building, which is wholly owned by Seward, represents a significant investment by the company following the securing of a NatWest/RBS Manufacturing Fund to ensure a doubling of manufacturing and storage facilities.
Seward Ltd, manufacturer and developer of the world leading, patented Stomacher® Laboratory Paddle Blender range for over 40 years, has moved to new premises within Worthing, UK. The new building, which is wholly owned by Seward, represents a significant investment by the company following the securing of a NatWest/RBS Manufacturing Fund to ensure a doubling of manufacturing and storage facilities. Bio-Chem Fluidics celebrates 25 years of operation at the Pittcon 2011 Conference and Expo. The company will feature its new line of Electric Rotary Valves and Solenoid Isolation Valves, at booth 3027. The show will take place from March 14-17 at Georgia World Congress Center (Atlanta, Ga.).
Bio-Chem Fluidics celebrates 25 years of operation at the Pittcon 2011 Conference and Expo. The company will feature its new line of Electric Rotary Valves and Solenoid Isolation Valves, at booth 3027. The show will take place from March 14-17 at Georgia World Congress Center (Atlanta, Ga.). Micronic Europe has announced a new 1.4ml amber polypropylene storage tube that, used in conjunction with a secure screw cap, ensures the integrity of light sensitive biological samples even over long-term storage periods.
Micronic Europe has announced a new 1.4ml amber polypropylene storage tube that, used in conjunction with a secure screw cap, ensures the integrity of light sensitive biological samples even over long-term storage periods. Porvair Sciences has introduced a version of its popular MicroluteTM Solid Phase Extraction (SPE) microplate which provides a wide assortment of phase chemistries and sorbent loadings in a single plate making it ideally suited for method development. The mix of phase chemistries and sorbent loadings available in the Development MicroluteTM allows you to simply and rapidly screen for the optimal retention and selectivity required to achieve your sample preparation objectives.
Porvair Sciences has introduced a version of its popular MicroluteTM Solid Phase Extraction (SPE) microplate which provides a wide assortment of phase chemistries and sorbent loadings in a single plate making it ideally suited for method development. The mix of phase chemistries and sorbent loadings available in the Development MicroluteTM allows you to simply and rapidly screen for the optimal retention and selectivity required to achieve your sample preparation objectives. Thermo Fisher Scientific Inc., the world leader in serving science, today announced that it has developed a powerful method for the trace-level analysis of carbonyl compounds in a wide range of matrices. Carbonyl compounds, hazardous pollutants released from diverse sources including motor vehicles and industrial emissions, have been shown to have adverse effects on human health. This new UHPLC/UV method enables the separation, detection and quantitation of parts per billion (ppb) concentrations of low molecular weight carbonyls in complex samples, safeguarding human health and ensuring compliance with industry regulations
Thermo Fisher Scientific Inc., the world leader in serving science, today announced that it has developed a powerful method for the trace-level analysis of carbonyl compounds in a wide range of matrices. Carbonyl compounds, hazardous pollutants released from diverse sources including motor vehicles and industrial emissions, have been shown to have adverse effects on human health. This new UHPLC/UV method enables the separation, detection and quantitation of parts per billion (ppb) concentrations of low molecular weight carbonyls in complex samples, safeguarding human health and ensuring compliance with industry regulations At Laboratory Automation 2011 in Palm Springs (Jan 29 - Feb 2), TTP LabTech will present a range of posters covering its complete product range, including two demonstrating the novel applications of its LAB2LAB automated sample transport and management system and its mosquito® nanoliter liquid handler.
At Laboratory Automation 2011 in Palm Springs (Jan 29 - Feb 2), TTP LabTech will present a range of posters covering its complete product range, including two demonstrating the novel applications of its LAB2LAB automated sample transport and management system and its mosquito® nanoliter liquid handler. The AMSBIO range of Universal Reference Products (RNA, cDNA and Protein Lysate) has been expanded to include standards for studies involving humans as well as for animal species including Monkey, Dog, Mouse, Rat, and Chicken. These products provide ideal controls for high throughput PCR, RNA microarray and antibody array studies
The AMSBIO range of Universal Reference Products (RNA, cDNA and Protein Lysate) has been expanded to include standards for studies involving humans as well as for animal species including Monkey, Dog, Mouse, Rat, and Chicken. These products provide ideal controls for high throughput PCR, RNA microarray and antibody array studies Inimex Pharmaceuticals and SARomics Biostructures announce that they have achieved a scientific milestone in their collaboration to solve the structure of Inimex's Innate Defense Regulator (IDR) IMX942 in complex with its target, the ZZ domain of human p62 (sequestosome-1). No structures were previously available for this challenging protein domain. The crystal structure determined by SARomics Biostructures provides invaluable information that will assist the functional understanding of IMX942 and the development of the new generation of IDRs.
Inimex Pharmaceuticals and SARomics Biostructures announce that they have achieved a scientific milestone in their collaboration to solve the structure of Inimex's Innate Defense Regulator (IDR) IMX942 in complex with its target, the ZZ domain of human p62 (sequestosome-1). No structures were previously available for this challenging protein domain. The crystal structure determined by SARomics Biostructures provides invaluable information that will assist the functional understanding of IMX942 and the development of the new generation of IDRs. Tecan has launched a Gas Control Module (GCM) for its Infinite 200 PRO multimode microplate reader, creating one of the most comprehensive solutions on the market for cell-based assays. Variations in environmental conditions can lead to inconsistent and unreliable data for cell-based optical studies, due changes in the pH and color of the media during incubation.
Tecan has launched a Gas Control Module (GCM) for its Infinite 200 PRO multimode microplate reader, creating one of the most comprehensive solutions on the market for cell-based assays. Variations in environmental conditions can lead to inconsistent and unreliable data for cell-based optical studies, due changes in the pH and color of the media during incubation. JULABO's product series „Presto PLUS" offers highly dynamic temperature control systems for demanding, external temperature control. „Presto PLUS" systems are used primarily for the temperature control of jacketed reaction vessels, autoclaves and reactor systems.
JULABO's product series „Presto PLUS" offers highly dynamic temperature control systems for demanding, external temperature control. „Presto PLUS" systems are used primarily for the temperature control of jacketed reaction vessels, autoclaves and reactor systems. HORIBA Medical's Pentra SAfe secure access solution for remote analysis is now available for both the ABX Pentra 400 clinical chemistry analyser and ABX Pentra 80 haematology analyser - both ideal for remote ‘hot lab' locations. The Pentra SAfe solution provides remote Point-of-Care (POC) managers full, secure access to all software on these analysers as if they were standing in front of them
HORIBA Medical's Pentra SAfe secure access solution for remote analysis is now available for both the ABX Pentra 400 clinical chemistry analyser and ABX Pentra 80 haematology analyser - both ideal for remote ‘hot lab' locations. The Pentra SAfe solution provides remote Point-of-Care (POC) managers full, secure access to all software on these analysers as if they were standing in front of them Preclinical Oncology Services Limited (PRECOS), a leading pre-clinical research and development service provider with a specific focus on oncology, has signed a significant service agreement with a leading global pharmaceutical company Janssen Pharmaceutica N.V. (Janssen). The global contract will see PRECOS provide clinical tumour material, tumour model development, target validation and drug efficacy work to support Janssen's oncology drug discovery, development and biomarker programmes.
Preclinical Oncology Services Limited (PRECOS), a leading pre-clinical research and development service provider with a specific focus on oncology, has signed a significant service agreement with a leading global pharmaceutical company Janssen Pharmaceutica N.V. (Janssen). The global contract will see PRECOS provide clinical tumour material, tumour model development, target validation and drug efficacy work to support Janssen's oncology drug discovery, development and biomarker programmes. NuAire's CO2 AutoFlow Incubators offer the highest level of performance and dependability for optimum growth conditions; designed with the most advanced features available for cell culture research and laboratory work.
NuAire's CO2 AutoFlow Incubators offer the highest level of performance and dependability for optimum growth conditions; designed with the most advanced features available for cell culture research and laboratory work.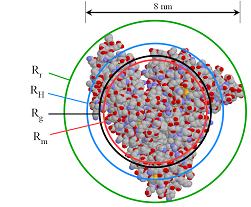 For many applications that include the use of biologically important molecules, such as proteins or polysaccharides, molecular size is an important parameter but its accurate measurement can be challenging. A new application note from Malvern Instruments describes the use of triple detection gel permeation/ size exclusion chromatography (GPC/SEC) using the VIscotek TDAmax system for this purpose.
For many applications that include the use of biologically important molecules, such as proteins or polysaccharides, molecular size is an important parameter but its accurate measurement can be challenging. A new application note from Malvern Instruments describes the use of triple detection gel permeation/ size exclusion chromatography (GPC/SEC) using the VIscotek TDAmax system for this purpose. CEVEC Pharmaceuticals , the developer of a novel human protein expression system derived from amniocytes, announced today the US expansion of it's business by founding a North American subsidiary, CEVEC Pharmaceuticals, Inc., in Potomac, Maryland, USA. Located close to Washington DC and amidst the Northeast US Bioscience Corridor, the incorporation of the new site reflects CEVEC ambition to better serve the growing US market.
CEVEC Pharmaceuticals , the developer of a novel human protein expression system derived from amniocytes, announced today the US expansion of it's business by founding a North American subsidiary, CEVEC Pharmaceuticals, Inc., in Potomac, Maryland, USA. Located close to Washington DC and amidst the Northeast US Bioscience Corridor, the incorporation of the new site reflects CEVEC ambition to better serve the growing US market. Thermo Fisher Scientific Inc., the world leader in serving science, today announced the new Thermo Scientific Sorvall RC 12BP Plus Centrifuge, a large-capacity, high-throughput floor standing centrifuge for blood banking and bioprocessing applications. With a maximum capacity of 12 L, the centrifuge can process up to 12 blood bag systems (up to 500 mL) or six 2000 mL Thermo Scientific Nalgene bio-bottles.
Thermo Fisher Scientific Inc., the world leader in serving science, today announced the new Thermo Scientific Sorvall RC 12BP Plus Centrifuge, a large-capacity, high-throughput floor standing centrifuge for blood banking and bioprocessing applications. With a maximum capacity of 12 L, the centrifuge can process up to 12 blood bag systems (up to 500 mL) or six 2000 mL Thermo Scientific Nalgene bio-bottles. Porvair Sciences has announced the MicroSeal - a new budget priced manual microplate heat sealer that offers great performance. Designed to meet the needs of low to medium throughput laboratories for microplate sealing the MicroSeal is compact and easy-to-use. An ergonomic pull down mechanism makes single action heat sealing of a wide range of plates quick and simple.
Porvair Sciences has announced the MicroSeal - a new budget priced manual microplate heat sealer that offers great performance. Designed to meet the needs of low to medium throughput laboratories for microplate sealing the MicroSeal is compact and easy-to-use. An ergonomic pull down mechanism makes single action heat sealing of a wide range of plates quick and simple. ITT Corporation (NYSE:ITT) announced that its Global Water Instrumentation brand, a leading provider of water monitoring equipment and part of the newly formed ITT Analytics, is introducing two new water quality sensors, the WQ-FDO Optical Dissolved Oxygen Sensor and the WQ-Cond Conductivity Sensor.
ITT Corporation (NYSE:ITT) announced that its Global Water Instrumentation brand, a leading provider of water monitoring equipment and part of the newly formed ITT Analytics, is introducing two new water quality sensors, the WQ-FDO Optical Dissolved Oxygen Sensor and the WQ-Cond Conductivity Sensor. Biotage (STO: BIOT), a leading supplier of tools and technology for medicinal and analytical chemistry, announced the second installment in its series of webinars focusing on peptide synthesis. The free events are in part a result of the research collaboration with Professor Knud J. Jensen at the Faculty of Life Sciences, University of Copenhagen, in developing new applications on the Biotage Syro WaveTM Parallel Peptide Synthesizer in the field of synthetic peptide and protein chemistry.
Biotage (STO: BIOT), a leading supplier of tools and technology for medicinal and analytical chemistry, announced the second installment in its series of webinars focusing on peptide synthesis. The free events are in part a result of the research collaboration with Professor Knud J. Jensen at the Faculty of Life Sciences, University of Copenhagen, in developing new applications on the Biotage Syro WaveTM Parallel Peptide Synthesizer in the field of synthetic peptide and protein chemistry. A new sample handling system from Beckman Coulter Inc., reduces minimum volume requirements on the Multisizer 4 COULTER COUNTER particle characterisation system from 10 mL to 4 mL.
A new sample handling system from Beckman Coulter Inc., reduces minimum volume requirements on the Multisizer 4 COULTER COUNTER particle characterisation system from 10 mL to 4 mL. Thermo Fisher Scientific Inc., the world leader in serving science, today announced a new robust, reliable method for analysis of trace elements in honey by atomic absorption. The Thermo Scientific iCE 3500 atomic absorption spectrometer offers a unique dual atomizer design which allows automatic, efficient and safe switching between flame and graphite furnace analyses without the need for user intervention or alignment adjustment.
Thermo Fisher Scientific Inc., the world leader in serving science, today announced a new robust, reliable method for analysis of trace elements in honey by atomic absorption. The Thermo Scientific iCE 3500 atomic absorption spectrometer offers a unique dual atomizer design which allows automatic, efficient and safe switching between flame and graphite furnace analyses without the need for user intervention or alignment adjustment. SAW Instruments GmbH has launched its unique sam5 biosensor instrument for advanced real-time biomolecular interaction and kinetic studies, at Biotechnica, Hannover, 5-7 October. The sam5 is a peerless biosensor utilising Surface Acoustic Wave technology for the label-free detection of real-time binding and structural events.
SAW Instruments GmbH has launched its unique sam5 biosensor instrument for advanced real-time biomolecular interaction and kinetic studies, at Biotechnica, Hannover, 5-7 October. The sam5 is a peerless biosensor utilising Surface Acoustic Wave technology for the label-free detection of real-time binding and structural events. Thermo Fisher Scientific Inc., the world leader in serving science, announces the launch of the online, fully-interactive version of its EP Scientific Products Catalog. As leading suppliers of clean sample containers for environmental and critical environment sampling EP Scientific cleans, packages and certifies its sample collection and storage containers in accordance with the EPA's regulatory guidelines.
Thermo Fisher Scientific Inc., the world leader in serving science, announces the launch of the online, fully-interactive version of its EP Scientific Products Catalog. As leading suppliers of clean sample containers for environmental and critical environment sampling EP Scientific cleans, packages and certifies its sample collection and storage containers in accordance with the EPA's regulatory guidelines. Choosing British designed and manufactured products will always be viewed as a better choice, especially for UK based companies and this certainly applies to the UK laboratory and life science sectors. Furthermore, when the environmental benefits, based on the increasingly important energy-efficiency comparisons are taken into account, the reasons for choosing British products become even more compelling
Choosing British designed and manufactured products will always be viewed as a better choice, especially for UK based companies and this certainly applies to the UK laboratory and life science sectors. Furthermore, when the environmental benefits, based on the increasingly important energy-efficiency comparisons are taken into account, the reasons for choosing British products become even more compelling