Channels
Special Offers & Promotions
LGC Standards
Products
Contact LGC Standards
All articles from LGC Standards
LGC Group and Dynamic Devices announce their strategic partnership to enhance genomic solutions
Apr 16, 2025
LGC acquires the Lipomed Reference Standards business, enhancing global portfolio of analytical reference materials
Apr 11, 2023
NML at LGC receives award to develop standards and deliver metrology training for engineering biology
Mar 7, 2023
The NML at LGC announces a major development in reference materials for allergen quantification
Feb 11, 2020
LGC acquires Toronto Research Chemicals, strengthening its presence in the reference standards market
Aug 13, 2019
LGC Accelerates the Development of New Medicines with Extractables and Leachables Services
Dec 6, 2016
Media Partners


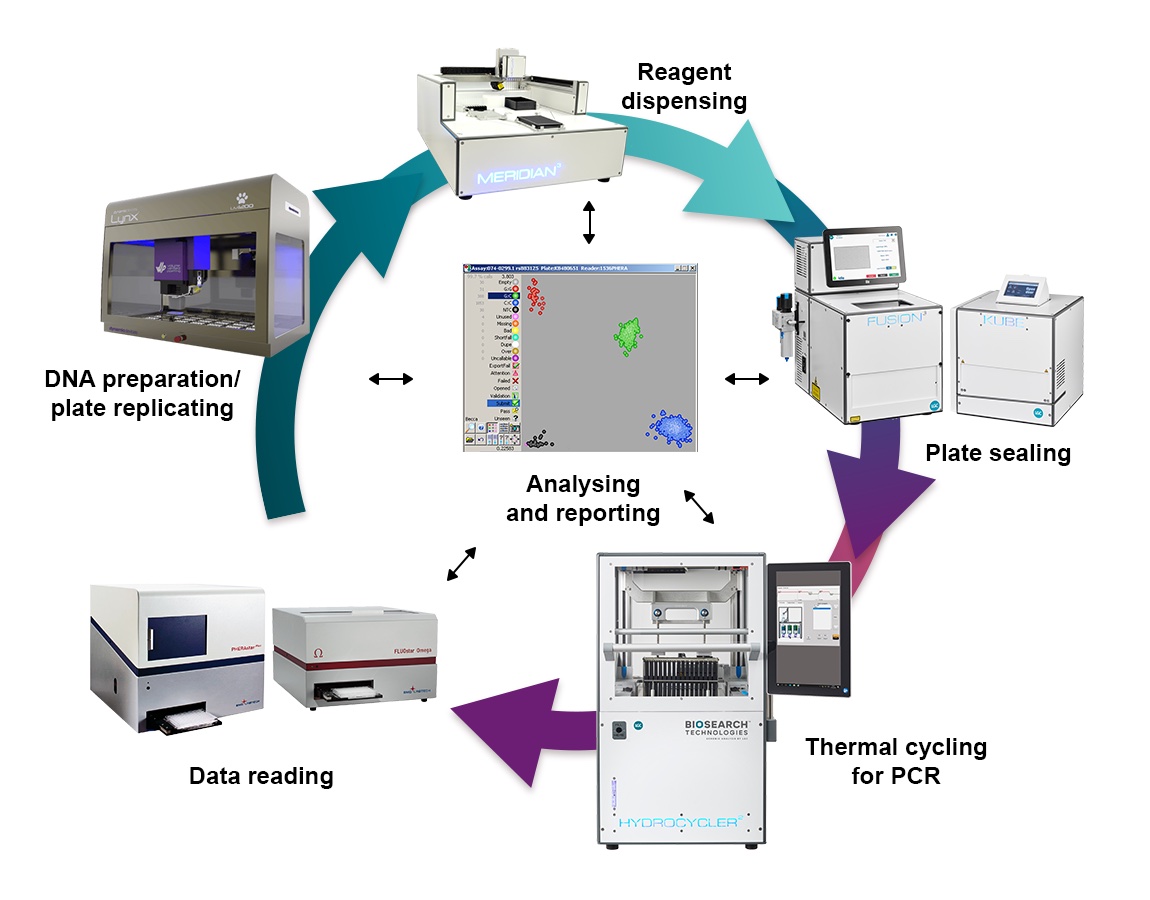 Biosearch Technologies, a part of LGC’s Diagnostics and Genomics division, and a global leader in high-throughput genotyping; and Dynamic Devices, a leader in automated liquid handling solutions announce their strategic partnership to provide workflow solutions for the global genomics market. By combining the innovative Lynx Robotic Liquid Handler with the SNPline™, sbeadex™ Lightning and Amp-Seq workflows, this partnership aims to deliver instruments, reagents, and consumables to service laboratories, breeders, and researchers worldwide...
Biosearch Technologies, a part of LGC’s Diagnostics and Genomics division, and a global leader in high-throughput genotyping; and Dynamic Devices, a leader in automated liquid handling solutions announce their strategic partnership to provide workflow solutions for the global genomics market. By combining the innovative Lynx Robotic Liquid Handler with the SNPline™, sbeadex™ Lightning and Amp-Seq workflows, this partnership aims to deliver instruments, reagents, and consumables to service laboratories, breeders, and researchers worldwide... LGC has announced the acquisition of Lipomed AG’s reference materials business including standards with drugs of abuse and toxicology applications, and thus greatly complementing LGC’s reference standards offering. Lipomed Reference Standards is a leading manufacturer and supplier of analytical reference standards with ISO/IEC 17025 accreditations for testing and ISO 17034 accreditation for production of reference materials...
LGC has announced the acquisition of Lipomed AG’s reference materials business including standards with drugs of abuse and toxicology applications, and thus greatly complementing LGC’s reference standards offering. Lipomed Reference Standards is a leading manufacturer and supplier of analytical reference standards with ISO/IEC 17025 accreditations for testing and ISO 17034 accreditation for production of reference materials... The National Measurement Laboratory (NML) at LGC has launched the Northern Cell Metrology Hub, a new centre of innovation for clinical diagnostics and medical technology, at the University of Leeds’ innovation community, Nexus. The first step in a partnership between the University of Leeds and the NML, the laboratory was launched on Friday, 31 March 2023 at a special event hosted Nexus, which features talks from Professors Julian Braybrook and Nick Plant, as well as a visit from the Mayor of West Yorkshire Tracy Brabin.
The National Measurement Laboratory (NML) at LGC has launched the Northern Cell Metrology Hub, a new centre of innovation for clinical diagnostics and medical technology, at the University of Leeds’ innovation community, Nexus. The first step in a partnership between the University of Leeds and the NML, the laboratory was launched on Friday, 31 March 2023 at a special event hosted Nexus, which features talks from Professors Julian Braybrook and Nick Plant, as well as a visit from the Mayor of West Yorkshire Tracy Brabin. The National Measurement Laboratory (NML) at LGC is pleased to announce that it has received an award from the Biotechnology and Biological Sciences Research Council (BBSRC) to develop standards and deliver metrology training for engineering biology. Engineering biology has the potential to offer solutions to a range of societal challenges, including food, biomedicines, clean growth and the environment...
The National Measurement Laboratory (NML) at LGC is pleased to announce that it has received an award from the Biotechnology and Biological Sciences Research Council (BBSRC) to develop standards and deliver metrology training for engineering biology. Engineering biology has the potential to offer solutions to a range of societal challenges, including food, biomedicines, clean growth and the environment... LGC Clinical Diagnostics, a leading supplier and partner to global in vitro diagnostics manufacturers and clinical laboratories, announce the extension of their exclusive partnership with Stanford University Department of Obstetrics & Gynecology - Maternal Fetal Medicine Division. Under the terms of the agreement, Stanford University will continue to provide LGC with clinical guidance in recruiting and selecting rare patient donors’ blood samples whose pregnancy has been identified with a genetic abnormality...
LGC Clinical Diagnostics, a leading supplier and partner to global in vitro diagnostics manufacturers and clinical laboratories, announce the extension of their exclusive partnership with Stanford University Department of Obstetrics & Gynecology - Maternal Fetal Medicine Division. Under the terms of the agreement, Stanford University will continue to provide LGC with clinical guidance in recruiting and selecting rare patient donors’ blood samples whose pregnancy has been identified with a genetic abnormality... A breakthrough in training for the cannabis testing industry has been launched thanks to a collaboration between experts in the National Measurement Laboratory (NML), hosted at LGC, and the reference materials division at LGC Standards. The rapid expansion of the global cannabis industry has led to sharply increased demand for safety and purity testing, but analytical laboratories are reporting a severe shortage of experienced cannabis testing staff...
A breakthrough in training for the cannabis testing industry has been launched thanks to a collaboration between experts in the National Measurement Laboratory (NML), hosted at LGC, and the reference materials division at LGC Standards. The rapid expansion of the global cannabis industry has led to sharply increased demand for safety and purity testing, but analytical laboratories are reporting a severe shortage of experienced cannabis testing staff... LGC announce the acquisition of Rapid Genomics, a provider of mid- to high-density Next-Generation Sequencing (NGS) kits and services for genotyping in the agrigenomics market. Rapid Genomics is a leading company in genotyping applications via Next-Generation Sequencing (NGS). Its Flex-Seq® Ex-L technology is provided to customers through kits and genotyping services, and allows high-throughput, cost-effective DNA analysis in plant, livestock and aquaculture genetics...
LGC announce the acquisition of Rapid Genomics, a provider of mid- to high-density Next-Generation Sequencing (NGS) kits and services for genotyping in the agrigenomics market. Rapid Genomics is a leading company in genotyping applications via Next-Generation Sequencing (NGS). Its Flex-Seq® Ex-L technology is provided to customers through kits and genotyping services, and allows high-throughput, cost-effective DNA analysis in plant, livestock and aquaculture genetics... LGC Maine Standards announces the release of VALIDATE® Thyroid 2 (THY2) to meet the linearity and calibration verification needs of clinical laboratories running the Beckman Coulter Unicel® DxI/Access platform. This exciting product, with the analyte Thyroglobulin (Tg), is formulated in human serum, using the CLSI EP06-A “equal delta” sample preparation method, and provides five distinct concentrations across the claimed and extended range of the Beckman Coulter Unicel® DxI/Access instrument...
LGC Maine Standards announces the release of VALIDATE® Thyroid 2 (THY2) to meet the linearity and calibration verification needs of clinical laboratories running the Beckman Coulter Unicel® DxI/Access platform. This exciting product, with the analyte Thyroglobulin (Tg), is formulated in human serum, using the CLSI EP06-A “equal delta” sample preparation method, and provides five distinct concentrations across the claimed and extended range of the Beckman Coulter Unicel® DxI/Access instrument... An international group of scientists, led by the National Measurement Laboratory for Chemical and Bio-Measurement team at LGC, has investigated quantitative reproducibility of SARS-CoV-2 analysis and how setting diagnostic cut-offs may alter the clinical sensitivity of COVID-19 testing. This analysis is vital to understanding potential discrepancies in measured viral burden used to manage the CoVid-19 pandemic....
An international group of scientists, led by the National Measurement Laboratory for Chemical and Bio-Measurement team at LGC, has investigated quantitative reproducibility of SARS-CoV-2 analysis and how setting diagnostic cut-offs may alter the clinical sensitivity of COVID-19 testing. This analysis is vital to understanding potential discrepancies in measured viral burden used to manage the CoVid-19 pandemic.... The Government Chemist (GC), hosted at LGC, has published the results of a recent international ring trial on the determination of cannabidiol (CBD) - an uncontrolled cannabinoid- and controlled cannabinoids in consumer products such as food and cosmetics. Consumer CBD products have entered the UK market rapidly in a variety of forms, including food and cosmetics. CBD when used as an ingredient in food or cosmetic products must currently be free from controlled cannabinoids....
The Government Chemist (GC), hosted at LGC, has published the results of a recent international ring trial on the determination of cannabidiol (CBD) - an uncontrolled cannabinoid- and controlled cannabinoids in consumer products such as food and cosmetics. Consumer CBD products have entered the UK market rapidly in a variety of forms, including food and cosmetics. CBD when used as an ingredient in food or cosmetic products must currently be free from controlled cannabinoids.... LGC has released ACCURUN® SARS-CoV-2 Antigen Reference Material Kit, a new quality measurement tool to support SARS-CoV-2 antigen testing. It is formulated for use with test methods that detect the nucleocapsid (NP) protein of SARS-CoV-2 virus – the target for the majority of the SARS-CoV-2 antigen assays currently on the market...
LGC has released ACCURUN® SARS-CoV-2 Antigen Reference Material Kit, a new quality measurement tool to support SARS-CoV-2 antigen testing. It is formulated for use with test methods that detect the nucleocapsid (NP) protein of SARS-CoV-2 virus – the target for the majority of the SARS-CoV-2 antigen assays currently on the market... LGC, Biosearch Technologies announce the release of a new set of ValuPanel™ Reagents to help the efforts of public health laboratories to detect SARS-CoV-2, influenza A, and influenza B. The CDC Influenza SARS-CoV-2 (Flu SC2) Multiplex ValuPanel Reagents - which are for research use only and not for use in diagnostic procedures - consists of probes and primers for multiplex RT-PCR detection and allows discrimination of SARS-CoV-2, influenza A, and influenza B from a single patient sample....
LGC, Biosearch Technologies announce the release of a new set of ValuPanel™ Reagents to help the efforts of public health laboratories to detect SARS-CoV-2, influenza A, and influenza B. The CDC Influenza SARS-CoV-2 (Flu SC2) Multiplex ValuPanel Reagents - which are for research use only and not for use in diagnostic procedures - consists of probes and primers for multiplex RT-PCR detection and allows discrimination of SARS-CoV-2, influenza A, and influenza B from a single patient sample.... LGC has expanded its innovative portfolio of SARS-CoV-2 quality solutions with the release of AccuPlex™ SARS-CoV-2 in Synthetic Oral Fluid reference material. The product is designed to support development and testing efforts around novel saliva-based SARS-CoV-2 diagnostics. It serves as an ideal research tool for assay developers as well as a complete quality solution for clinical laboratories employing such tests....
LGC has expanded its innovative portfolio of SARS-CoV-2 quality solutions with the release of AccuPlex™ SARS-CoV-2 in Synthetic Oral Fluid reference material. The product is designed to support development and testing efforts around novel saliva-based SARS-CoV-2 diagnostics. It serves as an ideal research tool for assay developers as well as a complete quality solution for clinical laboratories employing such tests.... LGC’s high-throughput (HTP) RT-PCR testing system for SARS-CoV-2 has been submitted to the FDA for Emergency Use Authorisation (EUA). Testing labs can now leverage LGC’s unrivalled expertise in automated sample preparation and high-throughput RT-PCR workflows in a ready-to-deploy solution. As the need for SARS-CoV-2 testing for both diagnostic and screening applications continues to grow, many organisations are looking to expand or create testing capacity...
LGC’s high-throughput (HTP) RT-PCR testing system for SARS-CoV-2 has been submitted to the FDA for Emergency Use Authorisation (EUA). Testing labs can now leverage LGC’s unrivalled expertise in automated sample preparation and high-throughput RT-PCR workflows in a ready-to-deploy solution. As the need for SARS-CoV-2 testing for both diagnostic and screening applications continues to grow, many organisations are looking to expand or create testing capacity... LGC SeraCare continues to innovate its portfolio of products supporting SARS-CoV-2 diagnostics. Initially designed to target the published CDC and WHO consensus sequences, the new AccuPlex™ SARS-CoV-2 Verification Panel and Reference Material Kit are now available with full SARS-CoV-2 viral genome coverage. This expansion allows for compatibility with any SARS-CoV-2 assay target, while maintaining the safety features....
LGC SeraCare continues to innovate its portfolio of products supporting SARS-CoV-2 diagnostics. Initially designed to target the published CDC and WHO consensus sequences, the new AccuPlex™ SARS-CoV-2 Verification Panel and Reference Material Kit are now available with full SARS-CoV-2 viral genome coverage. This expansion allows for compatibility with any SARS-CoV-2 assay target, while maintaining the safety features.... LGC has launched a Proficiency Testing (PT) service for the detection of SARS-CoV-2 (COVID 19) by nucleic acid amplification testing, one of the first global schemes for COVID-19. As increasing numbers of diagnostic methods are made available in the global fight against COVID 19, it is essential that laboratories ensure that their application of methods and subsequent results are reliable and accurate...
LGC has launched a Proficiency Testing (PT) service for the detection of SARS-CoV-2 (COVID 19) by nucleic acid amplification testing, one of the first global schemes for COVID-19. As increasing numbers of diagnostic methods are made available in the global fight against COVID 19, it is essential that laboratories ensure that their application of methods and subsequent results are reliable and accurate... LGC has has announced the acquisition of C/D/N Isotopes (CDN), a leading manufacturer and supplier of deuterium-labelled compounds. The acquisition follows LGC’s recent acquisition of Toronto Research Chemicals (TRC), the leading manufacturer and supplier of analytical standards, which has enjoyed a longstanding trading relationship with CDN....
LGC has has announced the acquisition of C/D/N Isotopes (CDN), a leading manufacturer and supplier of deuterium-labelled compounds. The acquisition follows LGC’s recent acquisition of Toronto Research Chemicals (TRC), the leading manufacturer and supplier of analytical standards, which has enjoyed a longstanding trading relationship with CDN.... LGC’s SeraCare Life Sciences, a manufacturer and leading partner to global in vitro diagnostic manufacturers and clinical laboratories, is announcing the development of molecular reference materials for coronavirus utilizing their proprietary AccuPlex™ recombinant technology. The control material is being produced in collaboration with several diagnostic manufacturers to support development, evaluation, and validation of molecular test methods directed to 2019-nCoV....
LGC’s SeraCare Life Sciences, a manufacturer and leading partner to global in vitro diagnostic manufacturers and clinical laboratories, is announcing the development of molecular reference materials for coronavirus utilizing their proprietary AccuPlex™ recombinant technology. The control material is being produced in collaboration with several diagnostic manufacturers to support development, evaluation, and validation of molecular test methods directed to 2019-nCoV.... The HD-X - the very latest fully automated technology platform from Quanterix for developing and performing digital immunoassays – has been unveiled at LGC’s GLP/GCP-accredited lab in Fordham, near Cambridge, UK. The HD-X builds on the success of the HD-1 analyser in conveying unprecedented levels of sensitivity, robustness, and throughput to Pharmacokinetic-Pharmacodynamic (PK-PD) bioanalytical studies....
The HD-X - the very latest fully automated technology platform from Quanterix for developing and performing digital immunoassays – has been unveiled at LGC’s GLP/GCP-accredited lab in Fordham, near Cambridge, UK. The HD-X builds on the success of the HD-1 analyser in conveying unprecedented levels of sensitivity, robustness, and throughput to Pharmacokinetic-Pharmacodynamic (PK-PD) bioanalytical studies.... LGC has announced the acquisition of a majority stake in Toronto Research Chemicals (“TRC”), a leading manufacturer and supplier of synthetic organic bio-chemicals which are used as reference standards, research tools and building blocks by a highly diversified global customer base across the pharmaceutical, applied and research sectors...
LGC has announced the acquisition of a majority stake in Toronto Research Chemicals (“TRC”), a leading manufacturer and supplier of synthetic organic bio-chemicals which are used as reference standards, research tools and building blocks by a highly diversified global customer base across the pharmaceutical, applied and research sectors... The Dr. Ehrenstorfer range now includes a growing number of reference standards manufactured under LGC’s ISO Guide 34 scope of accreditation, and new outer packaging and ampoules. The improved packaging, which uses cardboard with an upgraded cellophane layer to help prevent moisture uptake....
The Dr. Ehrenstorfer range now includes a growing number of reference standards manufactured under LGC’s ISO Guide 34 scope of accreditation, and new outer packaging and ampoules. The improved packaging, which uses cardboard with an upgraded cellophane layer to help prevent moisture uptake.... LGC, the international life sciences and measurement company, is successfully preventing pitfalls which can increase drug products time to market. There is increasing regulatory pressure on pharmaceutical companies to investigate the potential interaction between a dosage form and the primary closure and transfer system employed. A thorough scientifically sound extractables and leachables (E&L) programme is, therefore, an essential regulatory requirement...
LGC, the international life sciences and measurement company, is successfully preventing pitfalls which can increase drug products time to market. There is increasing regulatory pressure on pharmaceutical companies to investigate the potential interaction between a dosage form and the primary closure and transfer system employed. A thorough scientifically sound extractables and leachables (E&L) programme is, therefore, an essential regulatory requirement... Business Development Manager Martin Sibum explained: “We develop applications for a variety of markets, including clinical pharma, toxicology and forensics, and are involved in screening for drugs of abuse. The use of designer drugs is on the increase, and we needed to develop a quantitative online SPE-LC/MSMS method for the analysis of synthetic cannabinoids, or ‘Spice’ compounds. These compounds are relatively new, and LGC Standards was quite unique in being able to supply reference standards for five Spice compounds from stock.”...
Business Development Manager Martin Sibum explained: “We develop applications for a variety of markets, including clinical pharma, toxicology and forensics, and are involved in screening for drugs of abuse. The use of designer drugs is on the increase, and we needed to develop a quantitative online SPE-LC/MSMS method for the analysis of synthetic cannabinoids, or ‘Spice’ compounds. These compounds are relatively new, and LGC Standards was quite unique in being able to supply reference standards for five Spice compounds from stock.”... “Unilabs is a privately owned clinical laboratory that analyses company healthcare and workplace samples, as well as samples from drug abuse centres. Samples are received from across Sweden and screened either qualitatively or semi-quantitatively using immunological assays, followed by quantitative for verification. Ready-to-use LoGiCal solutions are ideal for our purposes; it is so much simpler than weighing powders for in¬house preparation of reference materials.”...
“Unilabs is a privately owned clinical laboratory that analyses company healthcare and workplace samples, as well as samples from drug abuse centres. Samples are received from across Sweden and screened either qualitatively or semi-quantitatively using immunological assays, followed by quantitative for verification. Ready-to-use LoGiCal solutions are ideal for our purposes; it is so much simpler than weighing powders for in¬house preparation of reference materials.”... Webshop registrations have increased by almost 300 % in the last four months. Customers can search the webshop quickly and easily, and enjoy the convenience and flexibility of being able to order 24 hours a day, 7 days a week.
Webshop registrations have increased by almost 300 % in the last four months. Customers can search the webshop quickly and easily, and enjoy the convenience and flexibility of being able to order 24 hours a day, 7 days a week.  LGC Standards, one of the world's leading suppliers of reference materials, has launched the LoGiCal range of certified reference materials. Manufactured and certified to ISO Guide 34, using ISO 17025 accredited processes in LGC's own laboratories, the new range offers scientists a choice of natural and deuterium labelled compounds, each supplied with a detailed Certificate of Analysis. LoGiCal certified reference materials fully satisfy national and international labelling and other requirements for transportation, health and safety...
LGC Standards, one of the world's leading suppliers of reference materials, has launched the LoGiCal range of certified reference materials. Manufactured and certified to ISO Guide 34, using ISO 17025 accredited processes in LGC's own laboratories, the new range offers scientists a choice of natural and deuterium labelled compounds, each supplied with a detailed Certificate of Analysis. LoGiCal certified reference materials fully satisfy national and international labelling and other requirements for transportation, health and safety... Over 14,500 products across a range of sectors including food, environmental and industry, to support analytical testing. LGC Standards' most comprehensive catalogue has been updated once again, with a wide range of new reference materials and suppliers being added to the existing offering. ‘Analytical reference materials, standards and high purity solvents 2011/12' supports laboratory quality by providing a resource that contains materials for a variety of applications, ensuring that users have all the products of interest in one place...
Over 14,500 products across a range of sectors including food, environmental and industry, to support analytical testing. LGC Standards' most comprehensive catalogue has been updated once again, with a wide range of new reference materials and suppliers being added to the existing offering. ‘Analytical reference materials, standards and high purity solvents 2011/12' supports laboratory quality by providing a resource that contains materials for a variety of applications, ensuring that users have all the products of interest in one place... LGC Standards is pleased to offer ULTRA Scientific's new line of ISO Guide 34 certified reference materials (CRM), ULTRAgold®. ULTRAgold fulfils international needs with CRMs at convenient concentrations of 1000 ppm and 10 ppm, as well as incorporating REACH (Registration, Evaluation, Authorisation and Restriction of Chemical Substances) labelling into its products, to better assist the laboratory community
LGC Standards is pleased to offer ULTRA Scientific's new line of ISO Guide 34 certified reference materials (CRM), ULTRAgold®. ULTRAgold fulfils international needs with CRMs at convenient concentrations of 1000 ppm and 10 ppm, as well as incorporating REACH (Registration, Evaluation, Authorisation and Restriction of Chemical Substances) labelling into its products, to better assist the laboratory community