Channels
Special Offers & Promotions
Synthetic Peptide Purification Using Preparative LC-MS
Synthetic Peptide Purification, focusing on purification by liquid chromatography-mass spectrometry (LC-MS).
Peptides are short chains of amino acids, typically less than 50 amino acids long. They exist in nature as signaling molecules, hormones, and neurotransmitters, but their use as therapeutics has grown tremendously in recent years.
Pharmaceutical peptides can be generated in a variety of ways. They can be generated by breaking down proteins to release peptides or they can be synthesized chemically, one amino acid at a time. Peptide synthesis produces the target peptide along with a mixture of impurities that must be removed before they are used therapeutically. For chemically synthesized peptides, purification requires meticulous methods because these impurities can be structurally similar to the desired product.
In this article Gilson covers synthetic peptide purification, focusing on purification by liquid chromatography-mass spectrometry (LC-MS).
Chemical Synthesis of Peptides - The Synthesis Cycle and Associated Impurities
Synthesizing peptides using chemical methods involves growing the peptide chain one amino acid at a time in the C-to-N direction (Figure 1). Purified amino acids used in synthesis contain protective groups that block functional groups on the amino acid from nonspecific reactions (e.g., blocking the N-terminal amine group or reactive side chains).
The first step in chemical synthesis is the removal of the protecting group from the solid support used to grow the peptide chain. Once the protecting group is removed, the first amino acid can be attached to the support and subsequently deprotected so that the next amino acid can be attached to the amino acid. This process repeats until all amino acid residues have been added to the chain. Finally, the remaining protection groups are removed from the peptide and the peptide can be cleaved from the solid support with a strong acid such as trifluoroacetic acid.
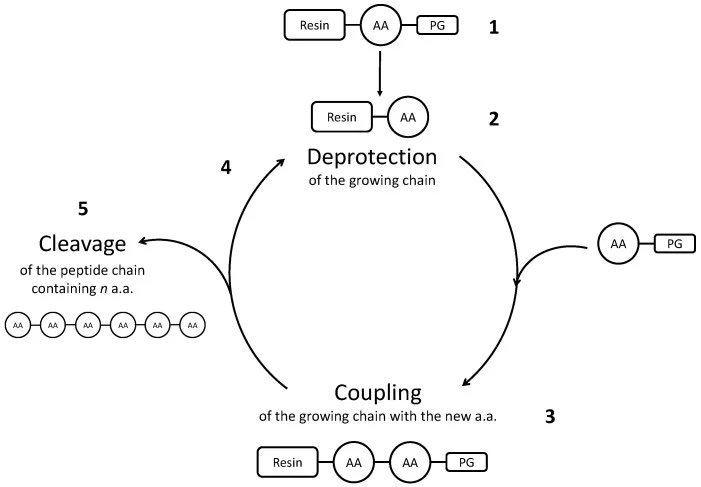
However, the protection, coupling, and deprotection reactions are not 100% efficient. If a protecting group is inadvertently removed at the wrong time, a side chain can form. If a protecting group was not removed from the N-terminus of the growing peptide chain during one cycle, this results in an incomplete peptide with missing amino acid residues. Therefore, the target peptide needs to be separated and purified from the impurities after synthesis.
Overview of Preparative LC-MS
Preparative LC-MS (liquid chromatography-mass spectrometry) is great for purifying peptides since it enables mass based targeting of the peptide of interest. Some impurities may be different from the target peptide by just one amino acid, making it difficult to distinguish them from the target peptide.
Prep LC-MS is a three-step process that involves:
- Separating the peptide mixture using HPLC: In LC-MS, reverse phase HPLC has been described as the “method of choice for peptide separation.” This separates molecules based on their hydrophobicity and their retention time on the column is an indication of hydrophobicity of the peptide.
- Confirming the identity of the compound using mass spectrometry: A small portion of the separated sample is diverted through the mass spectrometer to reveal its mass to charge properties.
- Collecting fractions that contain the target peptide: Once the mass spectrometer identifies the targeted ion(s) of the selected peptide, software such as Trilution LC will signal the fraction collector to collect the specified peptide.
Challenges to overcome: While trifluoroacetic acid (TFA) is a common additive to the mobile phase, it is considered to be an ion-suppressant, and it is therefore less attractive for purifications where on-line mass spectrometers are involved. Ion suppression can be bypassed when purifying on large columns using an active splitter and 0.1% formic acid solution as make-up solvent. Minimizing TFA from entering the mass spectrometer prolongs the equipment's lifetime, reduces maintenance, and avoids signal suppression.
Advantages of Prep LC-MS for Peptide Purification
Chromatography is the method of choice for peptide purification at the laboratory through manufacturing scale. Many biopharma processes use chromatography for both peptide capture and polishing. LC-MS has the added advantages below.

- Better separation of similar products. LC-MS can better separate target peptides from truncated products, incomplete peptides, and peptides that contain side-chain reactions.
- Fewer fractions collected. Compared to collecting based on UV signals, MS-based collection reduces the number of fractions collected significantly. This in turn reduces the number of samples that need to be processed further downstream in the workflow.
- Facilitates scale-up. Prep LC can be scaled up using different sized chromatography columns so it’s important to choose a system that can accommodate many column sizes if you plan to scale up. Read our case study about how Gilson’s preparative LC has helped scale up peptide purification here.
- Amenable to automation. LC-MS can be automated to increase throughput and reproducibility, freeing up your time for other tasks.
Gilson's preparative LC systems provide these benefits and more to give selectivity and versatility to peptide purification from lab scale to pilot scale. The VERITY® 271 LC-MS system couples HPLC with mass detection to guide fraction collection. It can be controlled using the TRILUTION® LC Software to provide real-time signal data.
Media Partners


