Channels
Special Offers & Promotions
New Application Note - Streamlined Purification of Assay-Ready Oligonucleotides by Automated HPLC
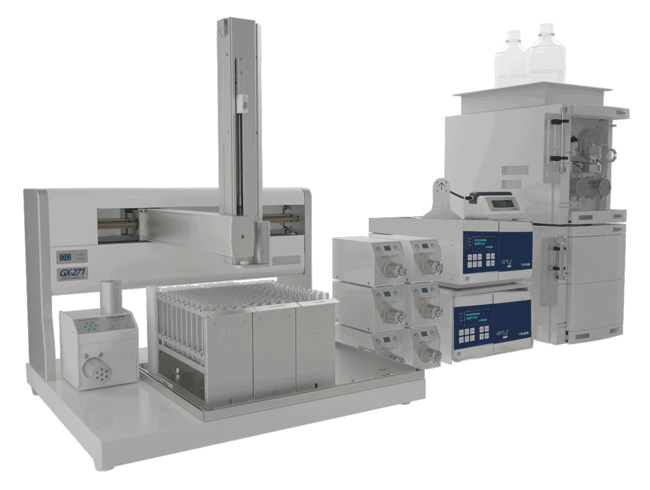
Gilson Applications Note AN1008
Oligonucleotides must be of high purity when used within molecular biology applications such as qPCR, next-generation sequencing, and microarray analysis
Custom manufactured oligonucleotides require additional purification steps after synthesis to separate the full‑length products from contaminants such as truncated sequences, base protecting groups, and other impurities. Efficient purification of oligonucleotides, of different lengths and at different production scales, presents a manufacturing challenge.
The GX‑271 Oligo Purification System, configured with automated preparative and analytical HPLC capabilities, can be used to isolate, analyze, and desalt reagent‑grade oligonucleotides in one continuous run. On‑board fraction analysis during runs enables on‑the‑fly sample addition resulting in no downtime on the instrument. Built‑in error handling conditions at various stages of the process prevent sample loss and assure full walk‑away capabilities.
In this application note, Gilson explore how the VERITY® Oligo Purification System, configured with automated preparative and analytical HPLC capabilities, can be a solution to isolate, analyze, and desalt reagent-grade oligonucleotides in one continuous run.

Media Partners


