Channels
Special Offers & Promotions
Cherwell Laboratories Ltd
Products
Contact Cherwell Laboratories Ltd
All articles from Cherwell Laboratories Ltd
Cherwell launches Redipor® TwistLock plates to enhance sample security in microbial environmental monitoring
Jun 10, 2025
Terminally sterilised plastic bottled media range launched to support sustainability initiatives
Jun 25, 2024
Cherwell launches Redipor® Beta Bags to support continuous manufacturing of sterile medicinal products
Jan 29, 2024
New study on microbial air sampling accuracy finds Petri dish dimensions can impact results
Jul 11, 2023
Cherwell to launch new portable Biofluorescent Particle Counter at Cleanroom Technology Conference UK
May 16, 2023
Cherwell to demonstrate new EM product pipeline at Pharmaceutical Microbiology Europe conference
Jan 5, 2023
Cherwell supports conference addressing challenges in aseptic manufacturing of advanced medicinal therapeutics
Aug 29, 2018
Cherwell to Participate in Key Annex 1 Sterile Medicinal Product Manufacture Conference
Feb 15, 2018
GOSH Selects Cherwell for Support in Excluding Bacterial Spores from Aseptic Compounding
Jul 20, 2017
Media Partners


 Cherwell (an AnalytiChem company), cleanroom microbiology solutions expert, is to be renamed AnalytiChem UK from the 1st September 2025. This represents the next step in Cherwell’s integration with the AnalytiChem Group, which it joined in January 2024. This transition is solely a change in name — all other aspects of Cherwell’s long-standing and trusted business, which flexibly and reliably delivers exceptionally high-quality products and services, will remain unchanged...
Cherwell (an AnalytiChem company), cleanroom microbiology solutions expert, is to be renamed AnalytiChem UK from the 1st September 2025. This represents the next step in Cherwell’s integration with the AnalytiChem Group, which it joined in January 2024. This transition is solely a change in name — all other aspects of Cherwell’s long-standing and trusted business, which flexibly and reliably delivers exceptionally high-quality products and services, will remain unchanged...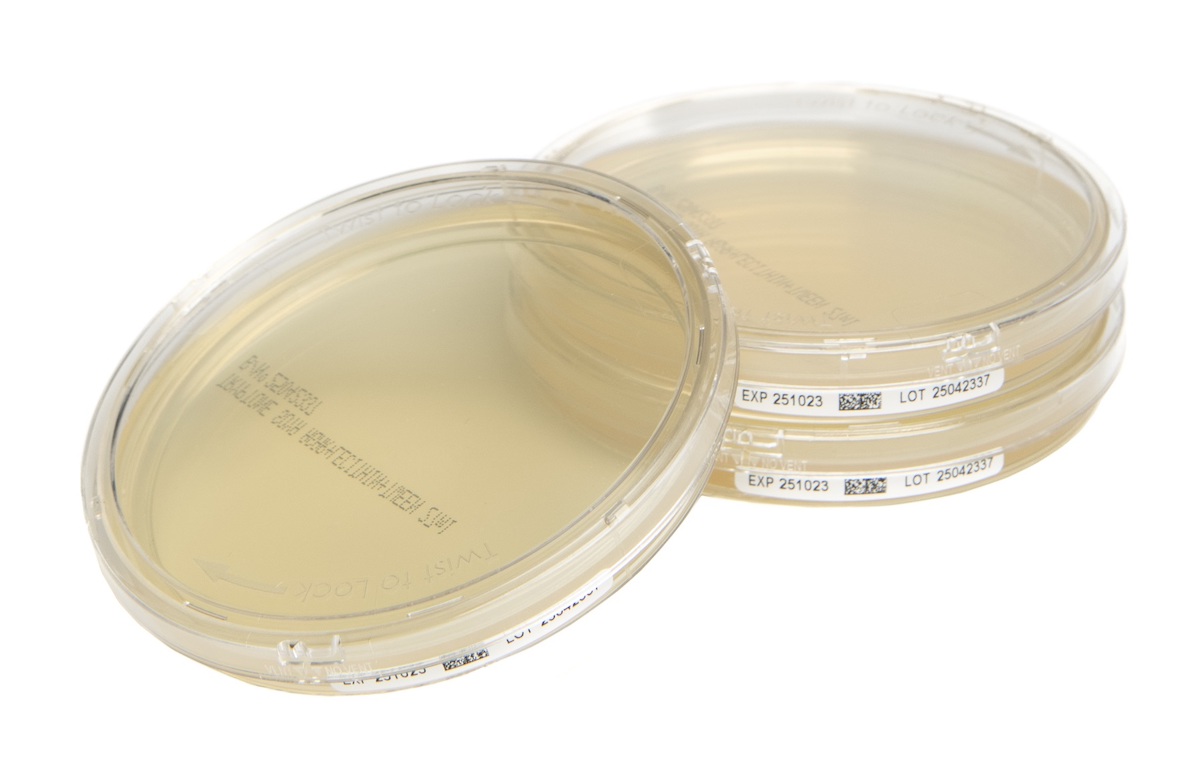 Cherwell (an Analytichem company), cleanroom microbiology solutions expert, has introduced a new range of plated prepared media designed to deliver sample security, data integrity, and optimised workflow in microbial monitoring. Cherwell’s new Redipor® TwistLock prepared media plates feature a secure locking lid and side-label with a GS1 DataMatrix barcode to enhance contamination protection, improve traceability and support automation, ensuring environmental monitoring (EM) meets exacting industry standards...
Cherwell (an Analytichem company), cleanroom microbiology solutions expert, has introduced a new range of plated prepared media designed to deliver sample security, data integrity, and optimised workflow in microbial monitoring. Cherwell’s new Redipor® TwistLock prepared media plates feature a secure locking lid and side-label with a GS1 DataMatrix barcode to enhance contamination protection, improve traceability and support automation, ensuring environmental monitoring (EM) meets exacting industry standards... Cherwell, cleanroom microbiology solutions expert, has introduced a new Redipor® Plastic Bottle prepared media range in response to a request from a large pharmaceutical company for support in meeting its sustainability targets. The new terminally sterilised plastic bottled media products have been developed and launched by Cherwell to offer a more environmentally friendly and cost-effective alternative...
Cherwell, cleanroom microbiology solutions expert, has introduced a new Redipor® Plastic Bottle prepared media range in response to a request from a large pharmaceutical company for support in meeting its sustainability targets. The new terminally sterilised plastic bottled media products have been developed and launched by Cherwell to offer a more environmentally friendly and cost-effective alternative...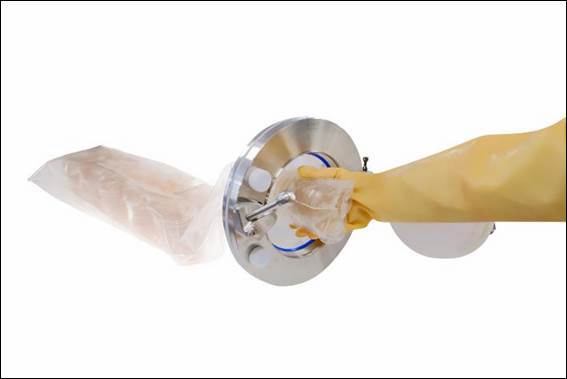 Cherwell, cleanroom microbiology solutions expert, has launched the Redipor® Beta Bag to ensure the safe transfer of ready-to-use gamma-irradiated prepared media into sterile manufacturing environments. This new multiple-use product transfer bag can reduce risk, cost and time in environmental monitoring (EM) processes during continuous manufacturing of sterile medicinal products. Offered as a tailored solution, customers can specify the media, formulation and plate type contained within Redipor Beta Bags, depending on their sterile workflow needs...
Cherwell, cleanroom microbiology solutions expert, has launched the Redipor® Beta Bag to ensure the safe transfer of ready-to-use gamma-irradiated prepared media into sterile manufacturing environments. This new multiple-use product transfer bag can reduce risk, cost and time in environmental monitoring (EM) processes during continuous manufacturing of sterile medicinal products. Offered as a tailored solution, customers can specify the media, formulation and plate type contained within Redipor Beta Bags, depending on their sterile workflow needs... Cherwell, cleanroom microbiology solutions expert, is excited to announce it is joining the AnalytiChem group of companies. Cherwell will join seven other businesses within the group, located across Europe, North America and Australia. Cherwell was founded in 1971 and serves customers across the UK and internationally with a range of products and solutions focused on environmental and process monitoring within pharmaceutical manufacturing...
Cherwell, cleanroom microbiology solutions expert, is excited to announce it is joining the AnalytiChem group of companies. Cherwell will join seven other businesses within the group, located across Europe, North America and Australia. Cherwell was founded in 1971 and serves customers across the UK and internationally with a range of products and solutions focused on environmental and process monitoring within pharmaceutical manufacturing... Cherwell, specialists in cleanroom microbiology solutions for the pharmaceutical, healthcare and related industries, has published findings of a new impartial investigation into the impact of using pre-poured Petri dishes from different suppliers for air sampling in cleanroom environmental monitoring (EM) programmes. This extends Cherwell’s initial ‘plate choice’ investigation which found that size and form factor of plates can affect microbial air sampling accuracy...
Cherwell, specialists in cleanroom microbiology solutions for the pharmaceutical, healthcare and related industries, has published findings of a new impartial investigation into the impact of using pre-poured Petri dishes from different suppliers for air sampling in cleanroom environmental monitoring (EM) programmes. This extends Cherwell’s initial ‘plate choice’ investigation which found that size and form factor of plates can affect microbial air sampling accuracy...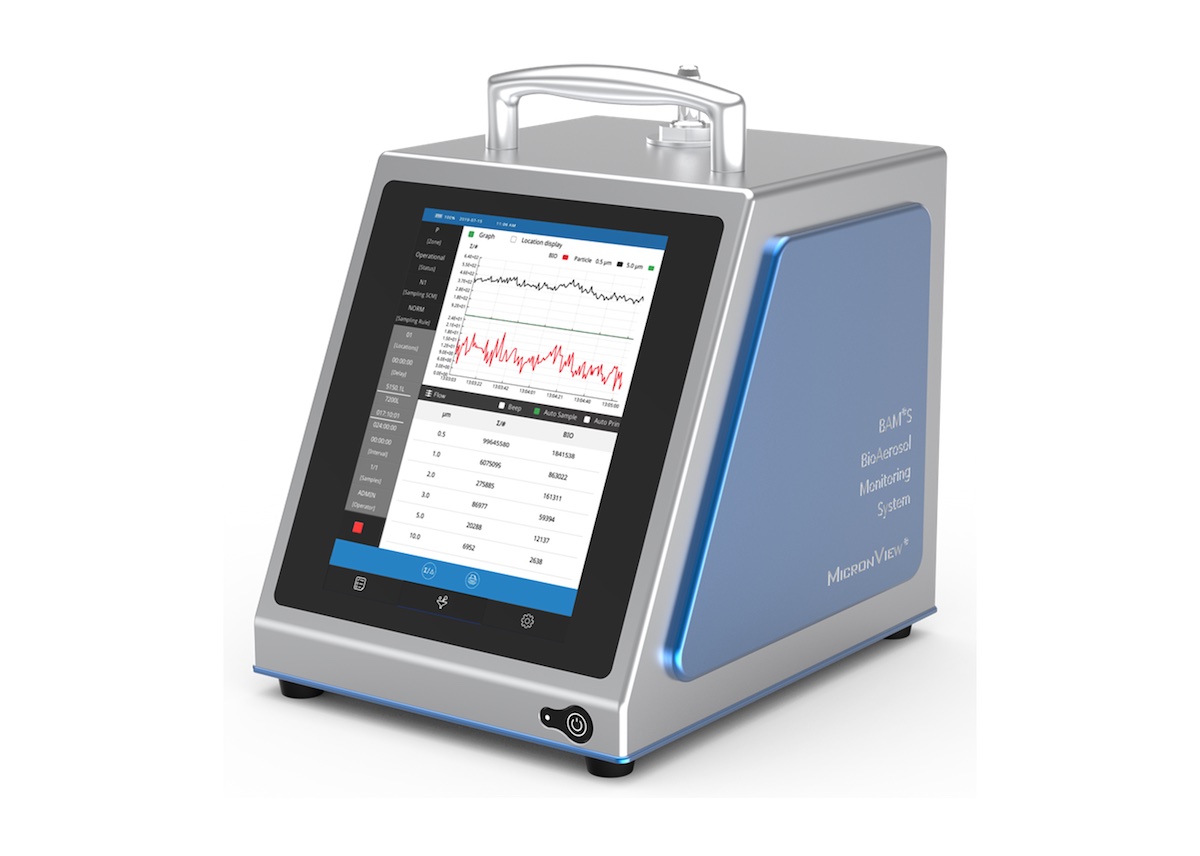 Cherwell, specialists in cleanroom microbiology solutions for the pharmaceutical, healthcare and related industries, will be launching a new portable Biofluorescent Particle Counter (BFPC) to the UK and Ireland at the Cleanroom Technology Conference 2023. On 24-25th May at Birmingham’s NCC on Stand E8, Cherwell will introduce the new MicronView BioAerosol Monitoring System (BAMS) which enables the rapid real-time, continuous monitoring of airborne microbes to support EU GMP Annex 1 requirements...
Cherwell, specialists in cleanroom microbiology solutions for the pharmaceutical, healthcare and related industries, will be launching a new portable Biofluorescent Particle Counter (BFPC) to the UK and Ireland at the Cleanroom Technology Conference 2023. On 24-25th May at Birmingham’s NCC on Stand E8, Cherwell will introduce the new MicronView BioAerosol Monitoring System (BAMS) which enables the rapid real-time, continuous monitoring of airborne microbes to support EU GMP Annex 1 requirements... Cherwell, specialists in cleanroom microbiology solutions for the pharmaceutical, healthcare and related industries, has published an update to its guide on “Environmental Monitoring Processes and Validation”, incorporating specific detail on the new version of EU GMP Annex 1. This aims to help sterile medicinal product manufacturers with reviewing and improving their Environmental Monitoring (EM) programs in preparation for compliance with the revised guidelines by August 2023...
Cherwell, specialists in cleanroom microbiology solutions for the pharmaceutical, healthcare and related industries, has published an update to its guide on “Environmental Monitoring Processes and Validation”, incorporating specific detail on the new version of EU GMP Annex 1. This aims to help sterile medicinal product manufacturers with reviewing and improving their Environmental Monitoring (EM) programs in preparation for compliance with the revised guidelines by August 2023... Cherwell, specialists in cleanroom microbiology solutions for the pharmaceutical, healthcare and related industries, will be exhibiting and discussing the latest additions to its high-quality range of environmental monitoring (EM) products at thePharmaceutical Microbiology Europe Conference, in London, 16-17 January 2023. This is the first time that Cherwell will attend this annual conference...
Cherwell, specialists in cleanroom microbiology solutions for the pharmaceutical, healthcare and related industries, will be exhibiting and discussing the latest additions to its high-quality range of environmental monitoring (EM) products at thePharmaceutical Microbiology Europe Conference, in London, 16-17 January 2023. This is the first time that Cherwell will attend this annual conference... Cherwell Laboratories, specialist supplier of environmental monitoring (EM) and process validation solutions for the pharmaceutical and related industries, has launched a new online video training library sharing educational content for individuals in the pharmaceutical and healthcare industries. Cherwell’s Delivering Knowledge platform offers best practice information delivered by experts in monitoring of controlled environments, aseptic processes and sterility...
Cherwell Laboratories, specialist supplier of environmental monitoring (EM) and process validation solutions for the pharmaceutical and related industries, has launched a new online video training library sharing educational content for individuals in the pharmaceutical and healthcare industries. Cherwell’s Delivering Knowledge platform offers best practice information delivered by experts in monitoring of controlled environments, aseptic processes and sterility... Cherwell Laboratories, specialist supplier of products for environmental monitoring and process validation, is proud to announce that it is celebrating 50 years since its founding by Lawrence Whittard in February 1971, the same year and month that the UK went decimal. To mark the Company’s significant milestone, Cherwell will be running a series of activities throughout its Golden Anniversary year...
Cherwell Laboratories, specialist supplier of products for environmental monitoring and process validation, is proud to announce that it is celebrating 50 years since its founding by Lawrence Whittard in February 1971, the same year and month that the UK went decimal. To mark the Company’s significant milestone, Cherwell will be running a series of activities throughout its Golden Anniversary year... Cherwell Laboratories, specialist supplier of products for environmental monitoring and process validation, has drawn on its extensive pharmaceutical and related industry knowledge to publish an eBook titled, “Growth Promotion Testing: A Guide to Good Practices” which is available to download from Cherwell’s website. The guide is intended for anyone involved in growth promotion testing of microbiological media, providing an overview of key considerations and best practice for this key quality control (QC) test...
Cherwell Laboratories, specialist supplier of products for environmental monitoring and process validation, has drawn on its extensive pharmaceutical and related industry knowledge to publish an eBook titled, “Growth Promotion Testing: A Guide to Good Practices” which is available to download from Cherwell’s website. The guide is intended for anyone involved in growth promotion testing of microbiological media, providing an overview of key considerations and best practice for this key quality control (QC) test... The user’s guide to regulatory expectations, the testing process, what to do in the event of test failure, and more. Cherwell Laboratories, specialist suppliers of products for environmental monitoring and process validation, has published an eBook titled, “Failure is not an option: Why sterility testing is so important”, which is available to download from Cherwell’s website....
The user’s guide to regulatory expectations, the testing process, what to do in the event of test failure, and more. Cherwell Laboratories, specialist suppliers of products for environmental monitoring and process validation, has published an eBook titled, “Failure is not an option: Why sterility testing is so important”, which is available to download from Cherwell’s website.... Easier navigation for environmental monitoring and sterilisation process validation product information with expert advice. Cherwell Laboratories, specialist suppliers ofenvironmental monitoringand process validation solutions for the pharmaceutical and related industries, announced the launch of its new website atwww.cherwell-labs.co.uk. The revamped website features a streamlined and simplified design, improved functionality and enriched content areas...
Easier navigation for environmental monitoring and sterilisation process validation product information with expert advice. Cherwell Laboratories, specialist suppliers ofenvironmental monitoringand process validation solutions for the pharmaceutical and related industries, announced the launch of its new website atwww.cherwell-labs.co.uk. The revamped website features a streamlined and simplified design, improved functionality and enriched content areas... Cherwell Laboratories, supplier of environmental monitoring and process validation products, has confirmed its continued support for the Pharmaceutical & Healthcare Sciences Society (PHSS) - UCL Q3P Annual Conference 2018. This year’s event will address the key challenges in manufacturing and aseptic processing in the new area of Biological and Advanced Therapy Medicinal Products (ATMPs), considering issues in GMP compliance and...
Cherwell Laboratories, supplier of environmental monitoring and process validation products, has confirmed its continued support for the Pharmaceutical & Healthcare Sciences Society (PHSS) - UCL Q3P Annual Conference 2018. This year’s event will address the key challenges in manufacturing and aseptic processing in the new area of Biological and Advanced Therapy Medicinal Products (ATMPs), considering issues in GMP compliance and... Further growth expected with announcement of new distributor in Bulgaria. Cherwell Laboratories, manufacturer of Redipor® prepared microbiological media, announced a 21% increase in Redipor sales to its distributors during its 2017/18 financial year. Cherwell’s continuing growth in export sales, largely across Europe, can be attributed to renewed strategic focus on overseas markets...
Further growth expected with announcement of new distributor in Bulgaria. Cherwell Laboratories, manufacturer of Redipor® prepared microbiological media, announced a 21% increase in Redipor sales to its distributors during its 2017/18 financial year. Cherwell’s continuing growth in export sales, largely across Europe, can be attributed to renewed strategic focus on overseas markets... UK & Euro versions of new Redipor price list released as customer base grows across Europe. Cherwell Laboratories, specialist suppliers of products for environmental monitoring and process validation, announces that a recent customer satisfaction survey has affirmed that the vast majority would recommend Cherwell and its Redipor®microbiological media products...
UK & Euro versions of new Redipor price list released as customer base grows across Europe. Cherwell Laboratories, specialist suppliers of products for environmental monitoring and process validation, announces that a recent customer satisfaction survey has affirmed that the vast majority would recommend Cherwell and its Redipor®microbiological media products... Joint PHSS & PQG event provides forum for revised sterile drug GMP guideline discussion. Cherwell Laboratories, specialist suppliers of products for environmental monitoring and process validation, will be exhibiting and participating in a key ‘EU GMP Annex 1 Draft Revision Insight’ meeting at Leamington Spa on 12th March. To be jointly hosted by the PHSS (Pharmaceutical & Healthcare Society) and the PQG (Pharmaceutical Quality Group), the event aims to provide a forum for discussion....
Joint PHSS & PQG event provides forum for revised sterile drug GMP guideline discussion. Cherwell Laboratories, specialist suppliers of products for environmental monitoring and process validation, will be exhibiting and participating in a key ‘EU GMP Annex 1 Draft Revision Insight’ meeting at Leamington Spa on 12th March. To be jointly hosted by the PHSS (Pharmaceutical & Healthcare Society) and the PQG (Pharmaceutical Quality Group), the event aims to provide a forum for discussion....
 Cherwell Laboratories, specialist suppliers of products for environmental monitoring, cleanroom bio-decontamination and process validation, has published a booklet titled, “The Pharmaceutical Lab’s Pocket Guide to Cleanroom Decontamination.”....
Cherwell Laboratories, specialist suppliers of products for environmental monitoring, cleanroom bio-decontamination and process validation, has published a booklet titled, “The Pharmaceutical Lab’s Pocket Guide to Cleanroom Decontamination.”.... Cherwell Laboratories, specialists in products for environmental monitoring and process validation, has recently supported the Great Ormond Street Hospital (GOSH) Pharmacy Unit in developing a new triple-wrapped prepared media product required to ensure the exclusion of bacterial spores during aseptic compounding validation....
Cherwell Laboratories, specialists in products for environmental monitoring and process validation, has recently supported the Great Ormond Street Hospital (GOSH) Pharmacy Unit in developing a new triple-wrapped prepared media product required to ensure the exclusion of bacterial spores during aseptic compounding validation.... Cherwell Laboratories, specialists in cleanroom microbiology solutions, have signed a distribution agreement with Pinpoint Scientific for the ImpactAir® range of microbial air samplers. The new ImpactAir range of high performance microbial air samplers has been designed to meet the demanding environmental monitoring requirements of aseptic processing in the pharmaceutical and healthcare industries....
Cherwell Laboratories, specialists in cleanroom microbiology solutions, have signed a distribution agreement with Pinpoint Scientific for the ImpactAir® range of microbial air samplers. The new ImpactAir range of high performance microbial air samplers has been designed to meet the demanding environmental monitoring requirements of aseptic processing in the pharmaceutical and healthcare industries.... Cherwell Laboratories is celebrating its 45th anniversary this November. The specialist suppliers of products for environmental monitoring, cleanroom bio-decontamination and process validation for healthcare, pharmaceutical and industrial applications was initially set up as a veterinary diagnostic laboratory in 1971 by Lawrence Whittard...
Cherwell Laboratories is celebrating its 45th anniversary this November. The specialist suppliers of products for environmental monitoring, cleanroom bio-decontamination and process validation for healthcare, pharmaceutical and industrial applications was initially set up as a veterinary diagnostic laboratory in 1971 by Lawrence Whittard... Cherwell Laboratories, specialists in cleanroom microbiology products, have introduced over 55 new products to their Redipor® prepared microbiological media range in the last twelve months. Cherwell’s dedication to customer service and their ability to deliver bespoke prepared media solutions has seen the company working closely with customers to deliver these new prepared media products to meet their specific...
Cherwell Laboratories, specialists in cleanroom microbiology products, have introduced over 55 new products to their Redipor® prepared microbiological media range in the last twelve months. Cherwell’s dedication to customer service and their ability to deliver bespoke prepared media solutions has seen the company working closely with customers to deliver these new prepared media products to meet their specific... Cherwell Laboratories
Cherwell Laboratories Cherwell Laboratories
Cherwell Laboratories Cherwell Laboratories
Cherwell Laboratories Cherwell Laboratories, specialists in cleanroom microbiology solutions, are pleased to announce the appointment of Harshad Joshi as Quality Manager. Harshad’s appointment marks Cherwell’s commitment to providing the highest quality products, enabling customers to retain complete confidence in the products Cherwell supply them with. Andy Whittard, Managing Director of Cherwell, commented, “We are delighted to welcome Harshad to Cherwell Laboratories. Harshad brings a wealth of...
Cherwell Laboratories, specialists in cleanroom microbiology solutions, are pleased to announce the appointment of Harshad Joshi as Quality Manager. Harshad’s appointment marks Cherwell’s commitment to providing the highest quality products, enabling customers to retain complete confidence in the products Cherwell supply them with. Andy Whittard, Managing Director of Cherwell, commented, “We are delighted to welcome Harshad to Cherwell Laboratories. Harshad brings a wealth of... Cherwell Laboratories, specialists in cleanroom microbiology solutions for the pharmaceutical and related industries, have announced the launch of a refreshed website. Designed to make it even easier for customers to find relevant product information, expert advice and technical information; the updated and fully responsive website also provides access to example product applications, testimonials and industry news via tablets and smartphones...
Cherwell Laboratories, specialists in cleanroom microbiology solutions for the pharmaceutical and related industries, have announced the launch of a refreshed website. Designed to make it even easier for customers to find relevant product information, expert advice and technical information; the updated and fully responsive website also provides access to example product applications, testimonials and industry news via tablets and smartphones... Cherwell Laboratories
Cherwell Laboratories Cherwell Laboratories, specialists in cleanroom microbiology products, have announced the availability of their 2015 Redipor® prepared microbiological media price list. The extensive Redipor range, manufactured at Cherwell’s ISO9001 registered site in Bicester offers ready-to-use media, including Petri dishes, contact plates, bottles and broth bags, designed to meet the rigorous requirements of the pharmaceutical and related industries...
Cherwell Laboratories, specialists in cleanroom microbiology products, have announced the availability of their 2015 Redipor® prepared microbiological media price list. The extensive Redipor range, manufactured at Cherwell’s ISO9001 registered site in Bicester offers ready-to-use media, including Petri dishes, contact plates, bottles and broth bags, designed to meet the rigorous requirements of the pharmaceutical and related industries... Initiated in response to a significant increase in demand, Cherwell has doubled the size of its cleanroom manufacturing suite and recruited additional staff to support the higher production capacity. Cherwell is renowned for offering a flexible supply of quality prepared media products, and the expanded facility ensures increasing demand in the UK and mainland Europe can be accommodated...
Initiated in response to a significant increase in demand, Cherwell has doubled the size of its cleanroom manufacturing suite and recruited additional staff to support the higher production capacity. Cherwell is renowned for offering a flexible supply of quality prepared media products, and the expanded facility ensures increasing demand in the UK and mainland Europe can be accommodated... Making Pharmaceuticals, to be held at NMM Exhibition Centre, Birmingham is the only event in the UK dedicated to the detailed and complex issues associated with sourcing, manufacturing, outsourcing and delivering consistent pharmaceutical products to the market. Experts from Cherwell Laboratories will be available throughout the 2-day event to discuss environmental monitoring and process validation solutions to meet the specific requirements of the pharmaceutical industry.....
Making Pharmaceuticals, to be held at NMM Exhibition Centre, Birmingham is the only event in the UK dedicated to the detailed and complex issues associated with sourcing, manufacturing, outsourcing and delivering consistent pharmaceutical products to the market. Experts from Cherwell Laboratories will be available throughout the 2-day event to discuss environmental monitoring and process validation solutions to meet the specific requirements of the pharmaceutical industry..... Detailing Cherwell’s extensive range of ready-to-use culture media in bottles and plates, the new prices are available on request. Manufactured at Cherwell’s ISO9001 registered site in Bicester, Redipor prepared media offers a high-quality, flexible solution for environmental monitoring, sterility testing of products, operator validation and process validation....
Detailing Cherwell’s extensive range of ready-to-use culture media in bottles and plates, the new prices are available on request. Manufactured at Cherwell’s ISO9001 registered site in Bicester, Redipor prepared media offers a high-quality, flexible solution for environmental monitoring, sterility testing of products, operator validation and process validation....
 Synergy Health’s Applied Sterilisation Technologies (AST) laboratory services are using Cherwell Laboratories’ SAS Pinocchio compressed air and bottled gas sampler to deliver an enhanced service to its customers. Synergy’s microbiological laboratories offer a wide range of services within the medical device and pharmaceutical manufacturing industries, including environmental monitoring, sterilisation validation and bioburden analysis. The introduction of Cherwell’s SAS Pinocchio is enabling Synergy to help their customers meet regulatory requirements more effectively...
Synergy Health’s Applied Sterilisation Technologies (AST) laboratory services are using Cherwell Laboratories’ SAS Pinocchio compressed air and bottled gas sampler to deliver an enhanced service to its customers. Synergy’s microbiological laboratories offer a wide range of services within the medical device and pharmaceutical manufacturing industries, including environmental monitoring, sterilisation validation and bioburden analysis. The introduction of Cherwell’s SAS Pinocchio is enabling Synergy to help their customers meet regulatory requirements more effectively...



 Cherwell Laboratories is celebrating its 40th anniversary this November. Founded in 1971 by Lawrence Whittard as a veterinary diagnostic laboratory, Cherwell has evolved over the years to dedicate its products and services to environmental monitoring and validation of sterilisation processes. The company's fully integrated product range for microbiological and quality assurance applications includes Redipor prepared media, SAS microbial air samplers, DataTrace wireless datalogging systems, as well as the recently acquired UK distributorships for Raven and Apex Laboratories' biological indicators...
Cherwell Laboratories is celebrating its 40th anniversary this November. Founded in 1971 by Lawrence Whittard as a veterinary diagnostic laboratory, Cherwell has evolved over the years to dedicate its products and services to environmental monitoring and validation of sterilisation processes. The company's fully integrated product range for microbiological and quality assurance applications includes Redipor prepared media, SAS microbial air samplers, DataTrace wireless datalogging systems, as well as the recently acquired UK distributorships for Raven and Apex Laboratories' biological indicators...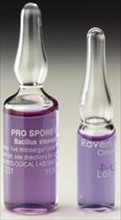 Cherwell Laboratories has taken over the UK distributorship for the Raven range of biological indicators, used for the validation of sterilisation processes. The comprehensive range includes a variety of biological indicators, such as spore strips and suspensions for use with conventional culture media, self-contained biological indicators and industrial use biological indicators. They are available populated by several different species of spores for applications including steam, dry heat, hydrogen peroxide vapour, Ethelyne Oxide (EO), formaldehyde, Chlorine Dioxide (ClO2) and gamma irradiation...
Cherwell Laboratories has taken over the UK distributorship for the Raven range of biological indicators, used for the validation of sterilisation processes. The comprehensive range includes a variety of biological indicators, such as spore strips and suspensions for use with conventional culture media, self-contained biological indicators and industrial use biological indicators. They are available populated by several different species of spores for applications including steam, dry heat, hydrogen peroxide vapour, Ethelyne Oxide (EO), formaldehyde, Chlorine Dioxide (ClO2) and gamma irradiation... Cherwell Laboratories, specialists in the manufacture of prepared microbiological media and supply of environmental monitoring instrumentation for pharmaceutical and related industries, has introduced paper-free labelling on all of its Redipor® products. All prepared media product labelling has now been migrated from paper to gloss white polypropylene labels to reduce risk of particulate contamination in sterile environments, such as cleanrooms and isolators, where Cherwell's products are frequently used...
Cherwell Laboratories, specialists in the manufacture of prepared microbiological media and supply of environmental monitoring instrumentation for pharmaceutical and related industries, has introduced paper-free labelling on all of its Redipor® products. All prepared media product labelling has now been migrated from paper to gloss white polypropylene labels to reduce risk of particulate contamination in sterile environments, such as cleanrooms and isolators, where Cherwell's products are frequently used... Cherwell Laboratories, specialists in the manufacture of prepared microbiological media and supply of environmental monitoring instrumentation for pharmaceutical and related industries, has introduced the Daily-Head disposable aspirating head for microbiological air monitoring. Triple-wrapped irradiated, this provides an always ready alternative to autoclaving stainless steel heads. Each batch of the Daily-Head is supplied with a certificate of sterilisation and conformity to satisfy cGLP requirements...
Cherwell Laboratories, specialists in the manufacture of prepared microbiological media and supply of environmental monitoring instrumentation for pharmaceutical and related industries, has introduced the Daily-Head disposable aspirating head for microbiological air monitoring. Triple-wrapped irradiated, this provides an always ready alternative to autoclaving stainless steel heads. Each batch of the Daily-Head is supplied with a certificate of sterilisation and conformity to satisfy cGLP requirements... Cherwell Laboratories, specialists in the manufacture of prepared microbiological media and supply of environmental monitoring instrumentation for pharmaceutical and related industries, has announced the availability of the 2011-2012 price list for its Redipor Prepared Media product portfolio...
Cherwell Laboratories, specialists in the manufacture of prepared microbiological media and supply of environmental monitoring instrumentation for pharmaceutical and related industries, has announced the availability of the 2011-2012 price list for its Redipor Prepared Media product portfolio... Cherwell Laboratories is to increase its facilities' footprint by over 60% to increase manufacturing and warehousing capacity for its range of environmental monitoring products. The opening of the new facility will mark Cherwell's 40th year and follows seven years of sustained positive company growth, including a 20% increase in export sales within the past financial year...
Cherwell Laboratories is to increase its facilities' footprint by over 60% to increase manufacturing and warehousing capacity for its range of environmental monitoring products. The opening of the new facility will mark Cherwell's 40th year and follows seven years of sustained positive company growth, including a 20% increase in export sales within the past financial year... Following its recent securing of a UK distribution agreement for DataTrace fully patented, high-tech data logging systems, Cherwell Laboratories has verified a rapid autoclave validation application. DataTrace are self-contained, wireless, high precision, data loggers designed specifically for use in critical manufacturing, quality control and validation applications.
Following its recent securing of a UK distribution agreement for DataTrace fully patented, high-tech data logging systems, Cherwell Laboratories has verified a rapid autoclave validation application. DataTrace are self-contained, wireless, high precision, data loggers designed specifically for use in critical manufacturing, quality control and validation applications. Following the previous successful installation of bespoke remote SAS microbial air samplers from Cherwell Laboratories, UK-based manufacturer of aseptically filled blood plasma products and part of NHSBT, Bio Products Laboratory (BPL) has now installed additional SAS samplers on a second plasma product aseptic filling line
Following the previous successful installation of bespoke remote SAS microbial air samplers from Cherwell Laboratories, UK-based manufacturer of aseptically filled blood plasma products and part of NHSBT, Bio Products Laboratory (BPL) has now installed additional SAS samplers on a second plasma product aseptic filling line Cherwell Laboratories, specialists in the manufacture of prepared microbiological media and supply of environmental monitoring instrumentation for pharmaceutical and related industries, has launched a new website to provide enhanced application and product information.
Cherwell Laboratories, specialists in the manufacture of prepared microbiological media and supply of environmental monitoring instrumentation for pharmaceutical and related industries, has launched a new website to provide enhanced application and product information.