Channels
Special Offers & Promotions
PerkinElmer
Products
Contact PerkinElmer
All articles from PerkinElmer
Launching Revvity: A Scientific Solutions Company Powering Innovation from Discovery to Cure
May 9, 2023
PerkinElmer Accelerates Transformation into High Growth, High Margin Life Sciences & Diagnostics Company
Aug 2, 2022
PerkinElmer Introduces New NGS Library Preparation Kits to Accelerate Research and Improve User Experience
Jun 28, 2022
Scitara announces collaboration with PerkinElmer Informatics to create new model of the modern lab
Apr 19, 2022
PerkinElmer Expands In Vivo Instruments Portfolio with Hands-free, High-throughput Vega
Apr 11, 2022
Carterra and PerkinElmer Sign Distribution Agreement For Asia-Pacific and Oceania Region for Carterra
Jan 4, 2022
PerkinElmer + Honeycomb Bio unveil a first-of-its-kind HIVE solution for single-cell profiling
Oct 20, 2021
UPM Biomedicals and PerkinElmer collaborate to offer high throughput 3D cell screening solution
Sep 30, 2021
PerkinElmer Expands KRAS Oncology Drug Discovery Assays with New Ready-to-Use AlphaLISA Kits
Aug 16, 2021
EUROIMMUN Launches Novel Ultrafast Automated Microscope and Intelligent Software for State-of-the-Art Diagnostics
Mar 16, 2021
Charnwood Lighthouse Lab Goes Live Processing Thousands of COVID-19 Tests per Day with PerkinElmer Solutions
Dec 1, 2020
PerkinElmer Brings ChemDraw Software to the Cloud, Enhancing Search and Collaboration Workflow
Nov 16, 2020
EUROIMMUN Launches Quantitative ELISA to Measure SARS-CoV-2 Antibodies Against Viral S1 Protein
Nov 10, 2020
PerkinElmer COVID-19 Test Kit Receives FDA Emergency Use Authorization for Sample Pooling
Nov 2, 2020
FDA Provides Emergency Use Authorization to PerkinElmer for Serological Test to Identify COVID-19 Antibodies
May 11, 2020
Beaumont Health launches largest serological testing study for COVID-19 antibodies in the USA
Apr 14, 2020
PerkinElmer Collaborates with Helix to Drive Innovation in Exome-Based Personal Genomics
Apr 25, 2018
Parent Project Muscular Dystrophy Selects PerkinElmer to Support Decode Duchenne Program
Apr 18, 2018
PerkinElmer Launches chemagic Prime Instrument for Automated Nucleic Acid Isolation and Assay Set-Up
Oct 31, 2017
PerkinElmer and In-Depth Genomics Team Up for Whole Genome Sequencing Diagnostic Program for Rare Diseases
Sep 29, 2017
PerkinElmer Display Laboratory Technologies for Environmental and Human Health Researchers at Analytica 2016
May 2, 2016
PerkinElmer Launches New OneSource Laboratory Consulting Services to Drive Improved Laboratory Efficiencies
May 30, 2013
PerkinElmer Showcases Latest Advancements in Thermal Instrumentation at Materials Research Society Meeting
Dec 12, 2012
Media Partners


 Covaris, a PerkinElmer company and pioneer in sample preparation technologies, and Hamilton Company, a global leader in automation solutions, announce a strategic partnership to co-market the truXTRAC® FFPE SMART Solution, powered by the Hamilton® Sonication STAR liquid handler. This joint solution offers a fully automated, walk-away workflow that delivers unmatched performance for nucleic acid extraction from FFPE samples...
Covaris, a PerkinElmer company and pioneer in sample preparation technologies, and Hamilton Company, a global leader in automation solutions, announce a strategic partnership to co-market the truXTRAC® FFPE SMART Solution, powered by the Hamilton® Sonication STAR liquid handler. This joint solution offers a fully automated, walk-away workflow that delivers unmatched performance for nucleic acid extraction from FFPE samples... The Medical Device Innovation Consortium (MDIC) has partnered with PerkinElmer's Horizon Discovery to develop and manufacture somatic reference samples (SRSs) to simplify and support validation of next generation sequencing (NGS)-based cancer diagnostics. The SRSs are expected to be commercially available by early 2024, following a rigorous characterization and validation process in collaboration with the National Institute for Standards and Technology (NIST)...
The Medical Device Innovation Consortium (MDIC) has partnered with PerkinElmer's Horizon Discovery to develop and manufacture somatic reference samples (SRSs) to simplify and support validation of next generation sequencing (NGS)-based cancer diagnostics. The SRSs are expected to be commercially available by early 2024, following a rigorous characterization and validation process in collaboration with the National Institute for Standards and Technology (NIST)...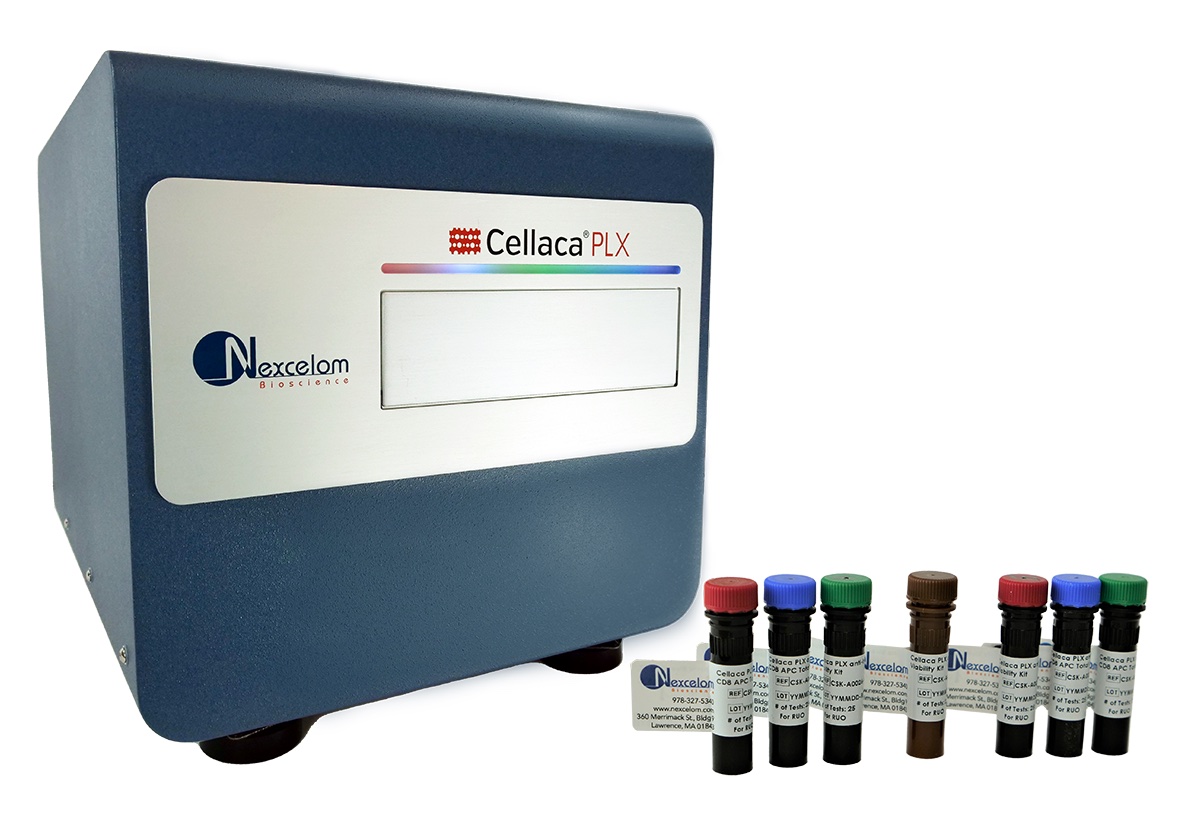 PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has launched the Cellaca® PLX Image Cytometry System, a first-of-its-kind benchtop platform that enables researchers to assess multiple Critical Quality Attributes (CQAs) of cell samples in a single automated workflow, including cell identity, quality and quantity. The cutting-edge Cellaca PLX system, designed by the company’s Nexcelom unit, combines best-in-class image cytometer hardware...
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has launched the Cellaca® PLX Image Cytometry System, a first-of-its-kind benchtop platform that enables researchers to assess multiple Critical Quality Attributes (CQAs) of cell samples in a single automated workflow, including cell identity, quality and quantity. The cutting-edge Cellaca PLX system, designed by the company’s Nexcelom unit, combines best-in-class image cytometer hardware... PerkinElmer’s Oxford Immunotec has announced the U.S. Food and Drug Administration (FDA) has approved the use of the T-Cell Select™ reagent kit for the automation of its T-SPOT®.TB test workflow for in vitro diagnostic (IVD) use by certified laboratories.The T-Cell Select reagent kit allows for a more automated workflow, designed to reduce hands-on time for lab personnel and lower overall costs...
PerkinElmer’s Oxford Immunotec has announced the U.S. Food and Drug Administration (FDA) has approved the use of the T-Cell Select™ reagent kit for the automation of its T-SPOT®.TB test workflow for in vitro diagnostic (IVD) use by certified laboratories.The T-Cell Select reagent kit allows for a more automated workflow, designed to reduce hands-on time for lab personnel and lower overall costs... PerkinElmer, Inc. has announced it has entered into an agreement with the intention to divest its Applied, Food and Enterprise Services businesses to New Mountain Capital, a leading growth-oriented private equity firm, for total consideration of $2.45 billion in cash, $2.30 billion of which will be received at the closing and $150 million of which will be payable contingent on the exit valuation New Mountain Capital receives on a sale or other capital events related to the business...
PerkinElmer, Inc. has announced it has entered into an agreement with the intention to divest its Applied, Food and Enterprise Services businesses to New Mountain Capital, a leading growth-oriented private equity firm, for total consideration of $2.45 billion in cash, $2.30 billion of which will be received at the closing and $150 million of which will be payable contingent on the exit valuation New Mountain Capital receives on a sale or other capital events related to the business... PerkinElmer has announced the launch of the research use only (RUO) BioQule™ NGS System – an automated benchtop solution for next-generation sequencing (NGS) library preparation of up to eight samples. By incorporating automated thermocycling, integrated quality control through optical quantification and robust liquid handling technology into a single device...
PerkinElmer has announced the launch of the research use only (RUO) BioQule™ NGS System – an automated benchtop solution for next-generation sequencing (NGS) library preparation of up to eight samples. By incorporating automated thermocycling, integrated quality control through optical quantification and robust liquid handling technology into a single device... PerkinElmer, Inc. (NYSE: PKI), a global leader committed to innovating for a healthier world, has announced the expansion of its in vivo imaging portfolio with the launch of the Vega® imaging system, a first-of-its kind ultrasound platform that combines hands-free, automated technology with high-throughput capability to accelerate non-invasive research and drug development studies of cancer, liver and kidney disease, cardiology and more.
PerkinElmer, Inc. (NYSE: PKI), a global leader committed to innovating for a healthier world, has announced the expansion of its in vivo imaging portfolio with the launch of the Vega® imaging system, a first-of-its kind ultrasound platform that combines hands-free, automated technology with high-throughput capability to accelerate non-invasive research and drug development studies of cancer, liver and kidney disease, cardiology and more. PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced V21 of its ChemDraw® software featuring the ability to import, animate and share 3D chemical structures natively in the Microsoft® PowerPoint® application with one click. The key enhancement, to a tool used by millions of scientists around the world, helps chemists create more intelligent research reports quickly and easily...
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced V21 of its ChemDraw® software featuring the ability to import, animate and share 3D chemical structures natively in the Microsoft® PowerPoint® application with one click. The key enhancement, to a tool used by millions of scientists around the world, helps chemists create more intelligent research reports quickly and easily... Carterra, Inc. the world leader in label-free high throughput antibody screening and characterization, has announced that it has signed an exclusive Asia-Pacific and Oceania region distribution agreement with PerkinElmer, Inc. a global leader committed to innovating for a healthier world. With the agreement, PerkinElmer will market, sell, and service Carterra’s flagship LSA high-throughput surface plasmon resonance (SPR) platform and software used for accelerating the discovery of therapeutic antibodies...
Carterra, Inc. the world leader in label-free high throughput antibody screening and characterization, has announced that it has signed an exclusive Asia-Pacific and Oceania region distribution agreement with PerkinElmer, Inc. a global leader committed to innovating for a healthier world. With the agreement, PerkinElmer will market, sell, and service Carterra’s flagship LSA high-throughput surface plasmon resonance (SPR) platform and software used for accelerating the discovery of therapeutic antibodies... PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced the launch of its PKeye™ Workflow Monitor, a cloud-based platform enabling laboratory personnel to remotely manage and monitor their PerkinElmer instruments and workflows in real-time. The PKeye Workflow Monitor offers scientists and researchers 24/7 access and visibility into their laboratory operations....
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced the launch of its PKeye™ Workflow Monitor, a cloud-based platform enabling laboratory personnel to remotely manage and monitor their PerkinElmer instruments and workflows in real-time. The PKeye Workflow Monitor offers scientists and researchers 24/7 access and visibility into their laboratory operations.... PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced the launch of its Signals Research™ Suite , a full cloud based solution, deployed on Amazon Web Services. The suite is a secure, informatics platform, providing integrated, end-to-end scientific data and workflow management for pharmaceutical and industrial customers. Designed to help drive more informed and accelerated decision making around drug, compound and formulation candidates...
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced the launch of its Signals Research™ Suite , a full cloud based solution, deployed on Amazon Web Services. The suite is a secure, informatics platform, providing integrated, end-to-end scientific data and workflow management for pharmaceutical and industrial customers. Designed to help drive more informed and accelerated decision making around drug, compound and formulation candidates... PerkinElmer, Inc. and Honeycomb Biotechnologies, Inc., announce the commercial launch of the first of its kind HIVE™ scRNAseq Solution for single-cell isolation and analysis. The HIVE solution leverages a portable, handheld device for the capture, storage and RNA-Seq library preparation of a diverse range of cell types, including fragile and labile cells such as granulocytes, nephrons, hepatocytes and neurons....
PerkinElmer, Inc. and Honeycomb Biotechnologies, Inc., announce the commercial launch of the first of its kind HIVE™ scRNAseq Solution for single-cell isolation and analysis. The HIVE solution leverages a portable, handheld device for the capture, storage and RNA-Seq library preparation of a diverse range of cell types, including fragile and labile cells such as granulocytes, nephrons, hepatocytes and neurons....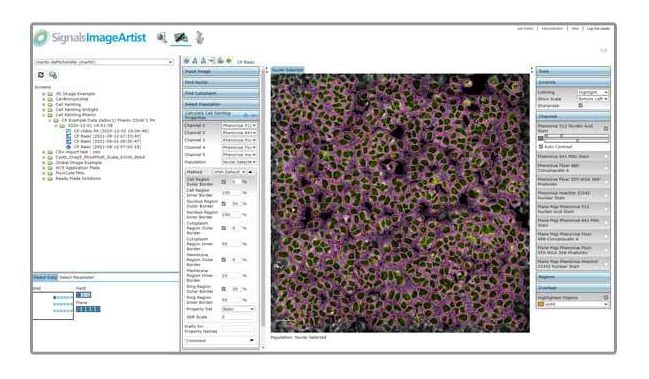 PerkinElmer, Inc., a global leading solutions provider in the life sciences, diagnostics, food and environmental testing markets, has announced the launch of Signals Image Artist™ software – a next-generation image analysis and management platform for drug discovery research. The new offering is designed to help scientists accurately process and analyse their high-content screening (HCS) and cellular imaging data in a matter of hours, instead of days or weeks, so they can make more informed decisions faster...
PerkinElmer, Inc., a global leading solutions provider in the life sciences, diagnostics, food and environmental testing markets, has announced the launch of Signals Image Artist™ software – a next-generation image analysis and management platform for drug discovery research. The new offering is designed to help scientists accurately process and analyse their high-content screening (HCS) and cellular imaging data in a matter of hours, instead of days or weeks, so they can make more informed decisions faster... UPM Biomedicals is pleased to announce that it has entered into an agreement with PerkinElmer Health Sciences, Inc., for the life sciences leader to act as a distributor of the GrowDex® and GrowDase™ range of products. This new collaboration will offer researchers a complete solution for high throughput screening (HTS) of 3D cell cultures in early drug discovery, bringing together PerkinElmer’s cell imaging and automation solutions and knowledge, and UPM’s animal-free 3D reagent offerings and expertise...
UPM Biomedicals is pleased to announce that it has entered into an agreement with PerkinElmer Health Sciences, Inc., for the life sciences leader to act as a distributor of the GrowDex® and GrowDase™ range of products. This new collaboration will offer researchers a complete solution for high throughput screening (HTS) of 3D cell cultures in early drug discovery, bringing together PerkinElmer’s cell imaging and automation solutions and knowledge, and UPM’s animal-free 3D reagent offerings and expertise...  PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced four new ready-to-use AlphaLISA® KRAS kits, designed to help scientists better understand complex KRAS protein structures and mutations so they can more easily, quickly, and precisely identify potential new therapeutic candidates for a wide range of prevalent cancers...
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced four new ready-to-use AlphaLISA® KRAS kits, designed to help scientists better understand complex KRAS protein structures and mutations so they can more easily, quickly, and precisely identify potential new therapeutic candidates for a wide range of prevalent cancers... Horizon Discovery, a PerkinElmer, Inc. company, announce that its gene editing and modulation portfolio is expanding to include a new family of CRISPR modulation (CRISPRmod) reagents for CRISPR interference (CRISPRi). CRISPRi enables scientists to better understand the biological pathways, processes and pathologies of disease by repressing genes at the transcriptional level, ultimately leading to new therapeutic approaches....
Horizon Discovery, a PerkinElmer, Inc. company, announce that its gene editing and modulation portfolio is expanding to include a new family of CRISPR modulation (CRISPRmod) reagents for CRISPR interference (CRISPRi). CRISPRi enables scientists to better understand the biological pathways, processes and pathologies of disease by repressing genes at the transcriptional level, ultimately leading to new therapeutic approaches.... EUROIMMUN, a PerkinElmer, Inc. company, announce the launch of the EUROPatternTM Microscope Live (EPML) compact immunofluorescence microscope, available with the fourth generation of the Company’s well-established EUROLabOfficeTM 4.0 (ELO 4.0) laboratory management software. The combined system of hardware and software allows for ultrafast automated immunofluorescence image acquisition, pattern recognition and titer estimation as well as modern diagnostics at the screen.
EUROIMMUN, a PerkinElmer, Inc. company, announce the launch of the EUROPatternTM Microscope Live (EPML) compact immunofluorescence microscope, available with the fourth generation of the Company’s well-established EUROLabOfficeTM 4.0 (ELO 4.0) laboratory management software. The combined system of hardware and software allows for ultrafast automated immunofluorescence image acquisition, pattern recognition and titer estimation as well as modern diagnostics at the screen. PerkinElmer Changes COVID-19 Rapid Testing Landscape with Highly Sensitive Point of Care Antigen Test for Mass Screening. PerkinElmer, Inc. has announced the launch of the PerkinElmer® COVID-19 Antigen Test for the qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in nasal (NS) or nasopharyngeal (NP) swab specimens. The lateral flow immunoassay test can be used to screen or to aid in diagnoses of COVID-19 in asymptomatic or symptomatic individuals....
PerkinElmer Changes COVID-19 Rapid Testing Landscape with Highly Sensitive Point of Care Antigen Test for Mass Screening. PerkinElmer, Inc. has announced the launch of the PerkinElmer® COVID-19 Antigen Test for the qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in nasal (NS) or nasopharyngeal (NP) swab specimens. The lateral flow immunoassay test can be used to screen or to aid in diagnoses of COVID-19 in asymptomatic or symptomatic individuals.... PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has launched the first cell painting kit as part of its new portfolio of PhenoVue™ cellular imaging reagents. This new range of reagents leverages PerkinElmer’s expertise in cellular imaging and high-content screening and works alongside the Company’s microplates, automation, software and industry-leading high-content screening instruments....
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has launched the first cell painting kit as part of its new portfolio of PhenoVue™ cellular imaging reagents. This new range of reagents leverages PerkinElmer’s expertise in cellular imaging and high-content screening and works alongside the Company’s microplates, automation, software and industry-leading high-content screening instruments.... "Transforming R&D: Digital innovation in the pharmaceuticals and chemicals industries," a new report by MIT Technology Review Insights, explores how leading pharmaceuticals and chemicals companies are using artificial intelligence, quantum computing, and other digital technologies to transform scientific research and enhance R&D performance. The report, produced in association with PerkinElmer Informatics, is based on in-depth interviews with R&D executives at organizations including Novartis, Roche, Merck, BASF, and Syngenta....
"Transforming R&D: Digital innovation in the pharmaceuticals and chemicals industries," a new report by MIT Technology Review Insights, explores how leading pharmaceuticals and chemicals companies are using artificial intelligence, quantum computing, and other digital technologies to transform scientific research and enhance R&D performance. The report, produced in association with PerkinElmer Informatics, is based on in-depth interviews with R&D executives at organizations including Novartis, Roche, Merck, BASF, and Syngenta.... PerkinElmer, Inc. has announced that its PerkinElmer® New Coronavirus Nucleic Acid Detection Kit received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) to test individuals without symptoms or other reasons to suspect COVID-19 infection. According to a new model from The Journal of the American Medical Association’s Open Network developed by researchers from the Centers for Disease Control and Prevention, close to 60% of total COVID-19 transmissions....
PerkinElmer, Inc. has announced that its PerkinElmer® New Coronavirus Nucleic Acid Detection Kit received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) to test individuals without symptoms or other reasons to suspect COVID-19 infection. According to a new model from The Journal of the American Medical Association’s Open Network developed by researchers from the Centers for Disease Control and Prevention, close to 60% of total COVID-19 transmissions.... Through this Acquisition, PerkinElmer will grow its portfolio of advanced infectious disease testing solutions to include tuberculosis detection to better serve customers around the world. Moreover, the deal will enable PerkinElmer to combine its channel expertise and leading workflow and testing capabilities with Oxford Immunotec’s leading proficiencies in T cell immunology with its proprietary test kits for latent tuberculosis....
Through this Acquisition, PerkinElmer will grow its portfolio of advanced infectious disease testing solutions to include tuberculosis detection to better serve customers around the world. Moreover, the deal will enable PerkinElmer to combine its channel expertise and leading workflow and testing capabilities with Oxford Immunotec’s leading proficiencies in T cell immunology with its proprietary test kits for latent tuberculosis.... PerkinElmer, Inc., announce that the Lighthouse Lab in Charnwood, Loughborough, England is now processing COVID-19 testing samples as part of the Company’s collaboration with the Department of Health and Social Care (DHSC) in support of the UK Government’s NHS Test and Trace program. The facility, operated by PerkinElmer Genomics, began processing samples and with its team of 400 scientists, researchers, technicians and operations personnel, has the capacity to test up to 50,000 samples daily by early 2021....
PerkinElmer, Inc., announce that the Lighthouse Lab in Charnwood, Loughborough, England is now processing COVID-19 testing samples as part of the Company’s collaboration with the Department of Health and Social Care (DHSC) in support of the UK Government’s NHS Test and Trace program. The facility, operated by PerkinElmer Genomics, began processing samples and with its team of 400 scientists, researchers, technicians and operations personnel, has the capacity to test up to 50,000 samples daily by early 2021.... PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has launched its newest ChemDraw® application, ChemOffice®+ Cloud, to simplify, facilitate and accelerate chemistry communication workflows. The ChemDraw platform is the leading chemistry drawing tool trusted around the world by scientists in commercial, government and academic lab settings across a number of research areas including life sciences, enviromental and materials....
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has launched its newest ChemDraw® application, ChemOffice®+ Cloud, to simplify, facilitate and accelerate chemistry communication workflows. The ChemDraw platform is the leading chemistry drawing tool trusted around the world by scientists in commercial, government and academic lab settings across a number of research areas including life sciences, enviromental and materials....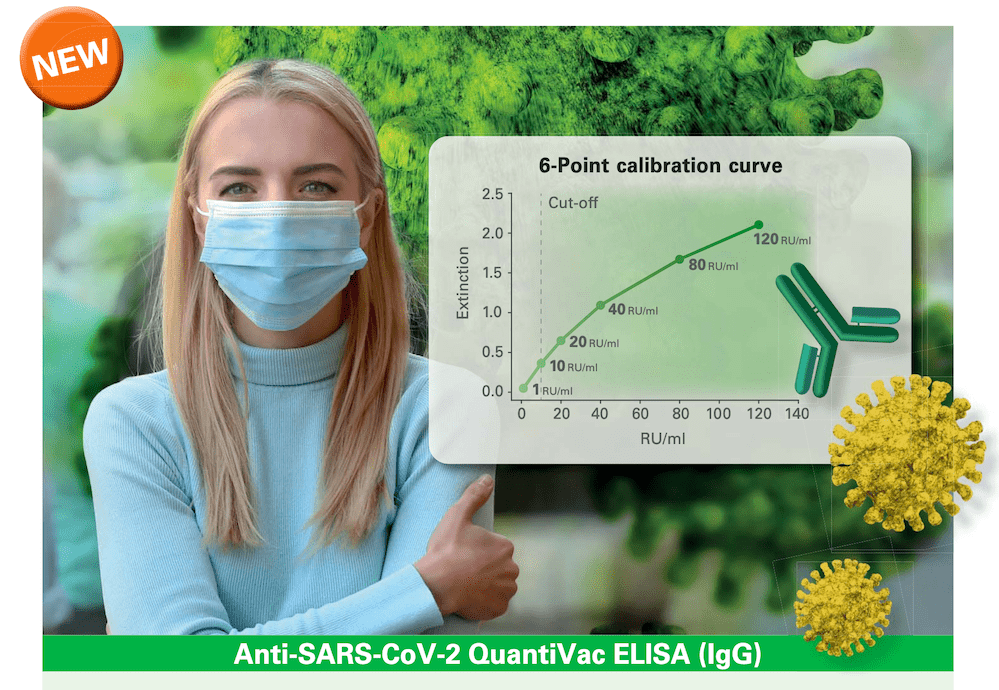 PerkinElmer, Inc., a global leader committed to innovating for a healthier world, announced today that EUROIMMUN, a PerkinElmer company, has launched the Anti-SARS-CoV-2 about ELISA (IgG) to quantify IgG antibodies against the SARS-CoV-2 S1 antigen. The assay is available for countries accepting the CE mark, and the Company plans to file a request for this product with the U.S. Food and Drug...
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, announced today that EUROIMMUN, a PerkinElmer company, has launched the Anti-SARS-CoV-2 about ELISA (IgG) to quantify IgG antibodies against the SARS-CoV-2 S1 antigen. The assay is available for countries accepting the CE mark, and the Company plans to file a request for this product with the U.S. Food and Drug... PerkinElmer, Inc. has announced that the U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) to allow sample pooling with PerkinElmer® New Coronavirus Nucleic Acid Detection Kit to increase the number of individuals who can be tested without increasing resources. This validation of batch testing using the industry’s most sensitive assay to date according to the
PerkinElmer, Inc. has announced that the U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) to allow sample pooling with PerkinElmer® New Coronavirus Nucleic Acid Detection Kit to increase the number of individuals who can be tested without increasing resources. This validation of batch testing using the industry’s most sensitive assay to date according to the  PerkinElmer, Inc.
PerkinElmer, Inc. PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced that the first Turnkey Lighthouse lab in Newport, South Wales, is now processing COVID-19 testing samples as part of the Company’s collaboration with the Department of Health and Social Care (DHSC) in support of the UK Government’s Test and Trace strategy...
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced that the first Turnkey Lighthouse lab in Newport, South Wales, is now processing COVID-19 testing samples as part of the Company’s collaboration with the Department of Health and Social Care (DHSC) in support of the UK Government’s Test and Trace strategy...
 PerkinElmer, Inc.
PerkinElmer, Inc. An advanced laboratory dedicated to leading Wales’ national Covid-19 testing has been established in Cardiff, thanks to collaboration between Public Health Wales and one of the world’s leading diagnostics companies. Creating a national Covid-19 testing lab at the University Hospital of Wales is an important part of the Welsh Government’s Test, Trace, Protect strategy and has been made possible thanks to the support...
An advanced laboratory dedicated to leading Wales’ national Covid-19 testing has been established in Cardiff, thanks to collaboration between Public Health Wales and one of the world’s leading diagnostics companies. Creating a national Covid-19 testing lab at the University Hospital of Wales is an important part of the Welsh Government’s Test, Trace, Protect strategy and has been made possible thanks to the support... PerkinElmer, Inc. is actively working with our global customers – which include specialty and reference diagnostic labs, clinics, hospitals, pharmaceutical and biopharmaceutical labs, academia, and governmental and research institutes – to combat the pandemic. Our innovative detection, workflow, and research solutions are being used to drive better outcomes as the world responds to COVID-19....
PerkinElmer, Inc. is actively working with our global customers – which include specialty and reference diagnostic labs, clinics, hospitals, pharmaceutical and biopharmaceutical labs, academia, and governmental and research institutes – to combat the pandemic. Our innovative detection, workflow, and research solutions are being used to drive better outcomes as the world responds to COVID-19.... U.S. Food and Drug Administration (FDA) has provided Emergency Use Authorization (EUA) for EUROIMMUN’s (a PerkinElmer company) Anti-SARS-CoV-2 ELISA (IgG) serology test. Clinical laboratories certified to perform high complexity tests under Clinical Laboratory Improvement Amendments (CLIA) can immediately begin using this ELISA for the detection of antibodies of the immunoglobulin class G....
U.S. Food and Drug Administration (FDA) has provided Emergency Use Authorization (EUA) for EUROIMMUN’s (a PerkinElmer company) Anti-SARS-CoV-2 ELISA (IgG) serology test. Clinical laboratories certified to perform high complexity tests under Clinical Laboratory Improvement Amendments (CLIA) can immediately begin using this ELISA for the detection of antibodies of the immunoglobulin class G.... Beaumont Health’s Research Institute is launching America’s largest serological testing study to help answer many questions surrounding the spread of COVID-19 and potentially help treat patients battling the virus. New study aims to determine the total population with COVID-19 antibodies across a health system, identify potential convalescent plasma donors, better understand disease transmission and contribute to a scientifically-based methodology for returning people to work....
Beaumont Health’s Research Institute is launching America’s largest serological testing study to help answer many questions surrounding the spread of COVID-19 and potentially help treat patients battling the virus. New study aims to determine the total population with COVID-19 antibodies across a health system, identify potential convalescent plasma donors, better understand disease transmission and contribute to a scientifically-based methodology for returning people to work.... Clinical laboratories certified under Clinical Laboratory Improvement Amendments (CLIA) can immediately begin using this kit to detect SARS-CoV-2, the virus that causes COVID-19. PerkinElmer’s RT-PCR test is marketed as an in vitro diagnostic (IVD) device by meeting the requirements of the European In Vitro Diagnostic Directive (IVDD) and is available in over 30 countries worldwide....
Clinical laboratories certified under Clinical Laboratory Improvement Amendments (CLIA) can immediately begin using this kit to detect SARS-CoV-2, the virus that causes COVID-19. PerkinElmer’s RT-PCR test is marketed as an in vitro diagnostic (IVD) device by meeting the requirements of the European In Vitro Diagnostic Directive (IVDD) and is available in over 30 countries worldwide.... PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has introduced new workstations and a high content screening system at SLAS2020 in San Diego. These technologies automate workflows so that disease research and drug discovery professionals can improve productivity, increase reproducibility, and garner key insights. PerkinElmer highlighted these new systems, along with several key offerings from its comprehensive portfolio of instruments and reagents....
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has introduced new workstations and a high content screening system at SLAS2020 in San Diego. These technologies automate workflows so that disease research and drug discovery professionals can improve productivity, increase reproducibility, and garner key insights. PerkinElmer highlighted these new systems, along with several key offerings from its comprehensive portfolio of instruments and reagents....
 Industry-Leading Adapters Mitigate Index Hopping and Spread of Signal in Next Gen Sequencing Applications. PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced its NEXTflex® unique dual index barcodes, the latest addition to the NEXTflex family of library prep solutions....
Industry-Leading Adapters Mitigate Index Hopping and Spread of Signal in Next Gen Sequencing Applications. PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced its NEXTflex® unique dual index barcodes, the latest addition to the NEXTflex family of library prep solutions.... PerkinElmer, Inc., has announced the launch of its chemagic™ Prime™ instrument, a new streamlined, walk-away sample processing solution which offers automated nucleic acid isolation and assay setup by combining PerkinElmer’s chemagic™ 360 instrument with the JANUS® automated liquid handling system....
PerkinElmer, Inc., has announced the launch of its chemagic™ Prime™ instrument, a new streamlined, walk-away sample processing solution which offers automated nucleic acid isolation and assay setup by combining PerkinElmer’s chemagic™ 360 instrument with the JANUS® automated liquid handling system.... PerkinElmer, Inc.has announced the launch of its QSight® 210 MD system, designed to provide routine high throughput quantitation to laboratories. The QSight 210 MD system includes tandem mass spectrometry (MSMS) technology designed to enable laboratories to run hundreds of samples per day....
PerkinElmer, Inc.has announced the launch of its QSight® 210 MD system, designed to provide routine high throughput quantitation to laboratories. The QSight 210 MD system includes tandem mass spectrometry (MSMS) technology designed to enable laboratories to run hundreds of samples per day.... Whether you’re performing ID testing or quantitative analysis for food, pharmaceuticals, polymers, or petroleum, you often need to test where the samples are – onsite, and on the fly. But you can’t sacrifice high performance for portability – which means your FT-NIR needs to deliver the same levels of sensitivity, resolution, and throughput, inside and outside the lab....
Whether you’re performing ID testing or quantitative analysis for food, pharmaceuticals, polymers, or petroleum, you often need to test where the samples are – onsite, and on the fly. But you can’t sacrifice high performance for portability – which means your FT-NIR needs to deliver the same levels of sensitivity, resolution, and throughput, inside and outside the lab....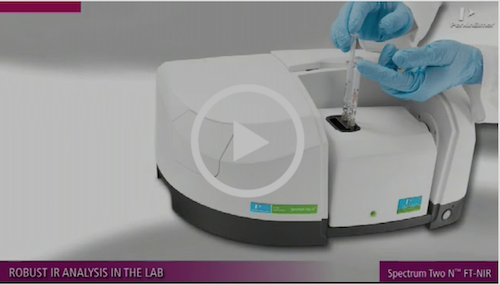 PerkinElmer, Inc., a global leader committed to innovating for a healthier world, today announced the launch of the Spectrum Two N™ FT-NIR system. This instrument is designed for laboratory technicians and staff conducting molecular spectroscopy analysis on a wide range of pharmaceutical, food and industrial samples....
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, today announced the launch of the Spectrum Two N™ FT-NIR system. This instrument is designed for laboratory technicians and staff conducting molecular spectroscopy analysis on a wide range of pharmaceutical, food and industrial samples.... All with the Avio 500 ICP-OES – a truly simultaneous, dual view, and compact ICP-OES. It utilizes a vertical plasma and is engineered to handle even the most difficult, high-matrix samples without dilution, delivering productivity, performance, and faster return on investment....
All with the Avio 500 ICP-OES – a truly simultaneous, dual view, and compact ICP-OES. It utilizes a vertical plasma and is engineered to handle even the most difficult, high-matrix samples without dilution, delivering productivity, performance, and faster return on investment.... PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced the launch of its new QSight™ Triple Quadrupole LC/MS/MS instrument at a press conference during analytica China, the international trade fair for laboratory technology, analysis, biotechnology and diagnostics held at the Shanghai New International Expo Centre....
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced the launch of its new QSight™ Triple Quadrupole LC/MS/MS instrument at a press conference during analytica China, the international trade fair for laboratory technology, analysis, biotechnology and diagnostics held at the Shanghai New International Expo Centre.... Magritek, a leading provider of compact NMR and MRI instruments, are pleased to announce further expansion in company activities worldwide. With offices now in the USA, Germany & New Zealand, the company has added experienced leaders in instrumentation sales and product marketing. With increased growth in worldwide markets for benchtop-sized analytical techniques, Magritek, leading providers of compact NMR and MRI systems, are pleased to be announcing the addition of two experienced industry professionals to their sales and marketing team...
Magritek, a leading provider of compact NMR and MRI instruments, are pleased to announce further expansion in company activities worldwide. With offices now in the USA, Germany & New Zealand, the company has added experienced leaders in instrumentation sales and product marketing. With increased growth in worldwide markets for benchtop-sized analytical techniques, Magritek, leading providers of compact NMR and MRI systems, are pleased to be announcing the addition of two experienced industry professionals to their sales and marketing team... PerkinElmer, Inc., a global leader in improving the health and safety of people and the environment, today introduced the first commercially available screening test for Severe Combined Immunodeficiency (SCID). The EnLite™ Neonatal TREC System expands the newborn screening portfolio of commercially available tests and will be introduced under CE marking, for sale in select countries in Europe and the Middle East. SCID impacts an estimated 1 in 50,000 to 1 in 100,000 newborns globally every year...
PerkinElmer, Inc., a global leader in improving the health and safety of people and the environment, today introduced the first commercially available screening test for Severe Combined Immunodeficiency (SCID). The EnLite™ Neonatal TREC System expands the newborn screening portfolio of commercially available tests and will be introduced under CE marking, for sale in select countries in Europe and the Middle East. SCID impacts an estimated 1 in 50,000 to 1 in 100,000 newborns globally every year... At the 2013 American Society for Mass Spectrometry Conference, PerkinElmer Inc., a global leader focused on the health and safety of people and the environment, introduced the AxION iQT gas chromatography tandem mass spectrometer (GC/MS/MS). The AxION iQT system provides greater quantitative capabilities to that of a triple quadrupole, while quickly identifying compounds like a quadrupole time-of-flight (Q-TOF) at a rate of up to 500 compounds per second. The new mass spectrometer provides laboratories with easier to use technology, faster results, and more specific, selective data needed to make more accurate decisions faster.....
At the 2013 American Society for Mass Spectrometry Conference, PerkinElmer Inc., a global leader focused on the health and safety of people and the environment, introduced the AxION iQT gas chromatography tandem mass spectrometer (GC/MS/MS). The AxION iQT system provides greater quantitative capabilities to that of a triple quadrupole, while quickly identifying compounds like a quadrupole time-of-flight (Q-TOF) at a rate of up to 500 compounds per second. The new mass spectrometer provides laboratories with easier to use technology, faster results, and more specific, selective data needed to make more accurate decisions faster..... PerkinElmer, Inc., a global leader focused on improving the health and safety of people and the environment, announced today that it is expanding its OneSource® Laboratory Services offering to include strategic laboratory consulting services. OneSource® Strategic Lab Solutions will deliver the combined power of PerkinElmer's expertise in informatics, instrumentation, service and process optimization, to increase laboratories' productivity and performance....
PerkinElmer, Inc., a global leader focused on improving the health and safety of people and the environment, announced today that it is expanding its OneSource® Laboratory Services offering to include strategic laboratory consulting services. OneSource® Strategic Lab Solutions will deliver the combined power of PerkinElmer's expertise in informatics, instrumentation, service and process optimization, to increase laboratories' productivity and performance....
 PerkinElmer, Inc., a global leader focused on improving the health and safety of people and the environment, today announced the introduction of the EnSpire® Multimode Plate Reader with Corning® Epic® label-free technology. This is the first and only benchtop detection platform to combine optical label-free technology and traditional labeled assays to accurately identify and characterize potential new therapeutic targets.
PerkinElmer, Inc., a global leader focused on improving the health and safety of people and the environment, today announced the introduction of the EnSpire® Multimode Plate Reader with Corning® Epic® label-free technology. This is the first and only benchtop detection platform to combine optical label-free technology and traditional labeled assays to accurately identify and characterize potential new therapeutic targets. PerkinElmer, Inc., a global leader focused on improving the health and safety of people and the environment, today announced the launch of its next generation DNA sequencing and data analysis services, to enable researchers to better explore the genomic origins of disease
PerkinElmer, Inc., a global leader focused on improving the health and safety of people and the environment, today announced the launch of its next generation DNA sequencing and data analysis services, to enable researchers to better explore the genomic origins of disease