Channels
Special Offers & Promotions
Groundbreaking Research Reveals Accuracy of PerkinElmer's High Volume NIPT Platform
 Study Results in Leading Scientific Publication Underscores Value of PerkinElmer’s Vanadis NIPT Solution
Study Results in Leading Scientific Publication Underscores Value of PerkinElmer’s Vanadis NIPT Solution
PerkinElmer, Inc., a global leader committed to innovating for a healthier world, has announced the publication of a research study describing the technology behind the Vanadis NIPT system. The article titled “Imaging Single DNA Molecules for High Precision NIPT” was published online on March 14 in Nature Scientific Reports.
Prenatal screening based on cell-free DNA (cfDNA), also referred to as non-invasive prenatal testing (NIPT), is increasingly becoming the standard follow-up procedure for patients classified as high risk based on traditional first trimester screening. While NIPT is becoming an accepted follow- up test for aneuploidy screening, cost and complexity remain key challenges to making NIPT available to a wider range of laboratories and women. This has limited the adoption of existing NIPT solutions to mainly specialized genetic laboratories, as well as hindered wider implementation of NIPT in contemporary screening programs.
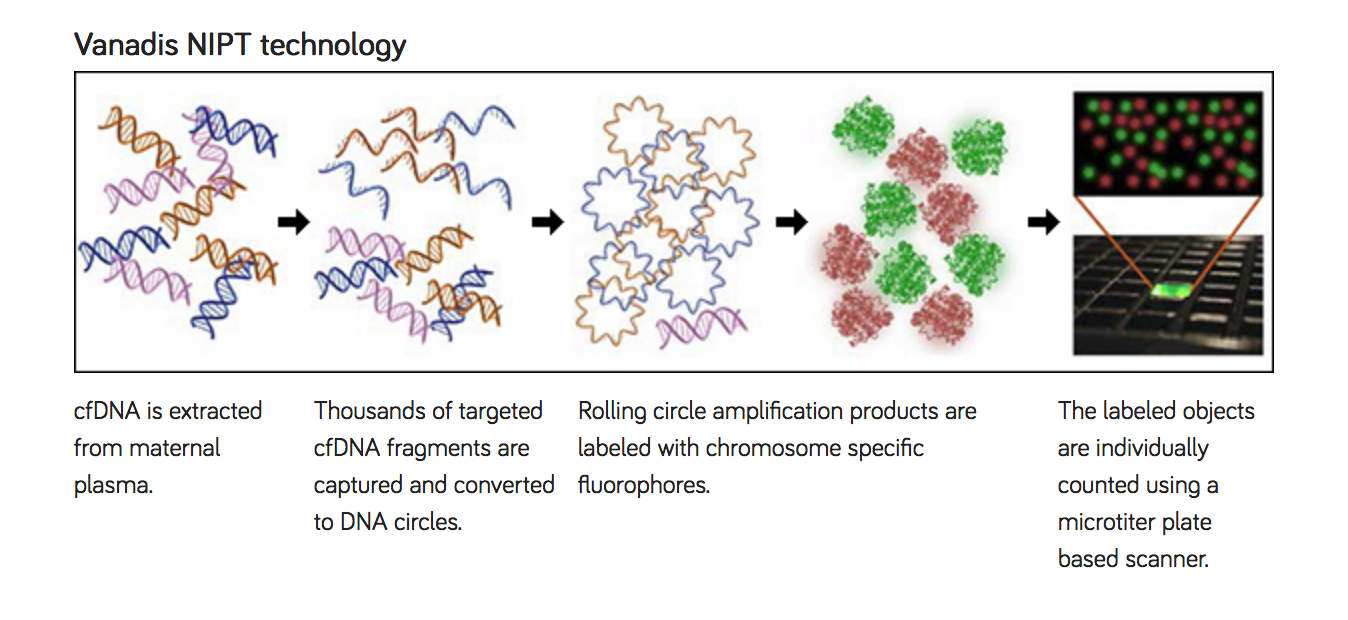
PerkinElmer’s Vanadis NIPT solution, currently under development, is a cost-effective, scalable platform for the measurement of fetal chromosomal trisomies in maternal plasma by labeling and counting specific cfDNA molecules using imaging. PerkinElmer obtained the proprietary technology through its acquisition of Vanadis Diagnostics, AB. The platform is designed for cost-effective high-throughput screening using NIPT. The assay provides automated testing from primary blood tubes to provide results. The Vanadis NIPT system does not depend on advanced genetic technologies such as sequencing or microarrays, and thereby reduces assay cost and complexity.
The study presented data demonstrating the feasibility of applying the Vanadis NIPT assay for detection of fetal trisomy 21 in maternal plasma. Furthermore, assessment of the Vanadis NIPT assay precision shows that reference samples with levels of fetal DNA at and below 4% can be analyzed and classified correctly. The results are generated using an assay that readily can be automated and do not depend on sequencing or microarrays. Instead, the readout system used is a dedicated version of PerkinElmer’s Operetta® high- content imaging product line. One advantage of using imaging instead of sequencing is the simplicity of the analysis. This is an important step toward making NIPT more broadly available to screening programs and pregnant women.
“As a global leader in prenatal screening for over 30 years, our Vanadis assay plays a critical role in offering cfDNA testing to more women in order to improve screening of fetal chromosomal abnormalities,” said Linh Hoang MD, PhD, Vice President, Reproductive Health, PerkinElmer.
“We developed the Vanadis technology to fundamentally change the cost structure and workflow for NIPT,” said Olle Ericsson, PhD, General Manager at Vanadis Diagnostics, a PerkinElmer company. “This study contains the first clinical proof of concept data, and we believe our Vanadis NIPT solution will have a dramatic impact in the effort to provide more women to access to NIPT in the near future.”
“Reducing costs and simplification of the analysis, to enable higher throughput of samples, are the key remaining barriers in order to increase access globally to cfDNA testing for aneuploidies. This study shows promising results towards achieving this goal” said Professor Kypros Nicolaides, one of the authors of the study and Director of Harris Birthright Research Centre for Fetal Medicine at King's College London and Chairman of the Fetal Medicine Foundation.
Media Partners