Channels
Special Offers & Promotions
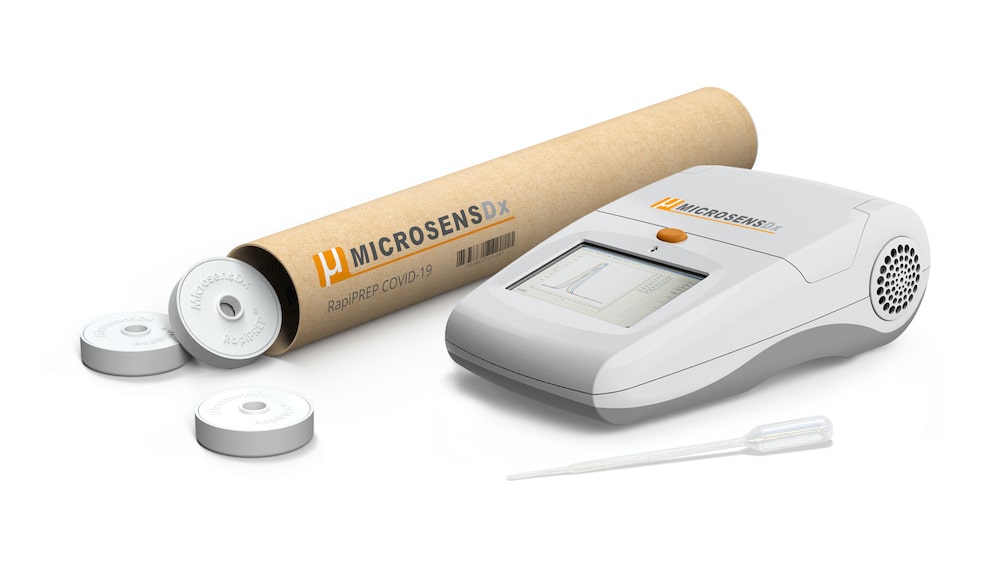
Breakthrough 25-minute COVID-19 Test set for Launch
A BREAKTHROUGH test for COVID-19 which works in just 25 minutes, with high accuracy and requiring only routine laboratory equipment, is currently in the final stages of clinical validation by British biotech firm MicrosensDx.
The test, called MicrosensDx COVID-19, has been used successfully in large scale testing programs with BAE Systems in Cumbria over the last few months, where it has been deployed to test more than 8,000 people a week. MicrosensDx is now planning to scale up for wider availability, both in the UK and across Europe.
Crucially, the test uses fewer of the globally limited reagents that other COVID-19 test technologies require and which have been in short supply across Europe in recent months.
It can be used within mass testing laboratories, but is also available for use in a ‘near patient’ mini-lab format, by healthcare staff with basic laboratory training, to enable rapid real-time testing. Crucially the result is available in less than a third of the time required by the widespread Public Health England PCR test [90 minutes].
The speed and ease of use presents clear potential benefit not only to frontline health and care workers but to any population or setting where a rapid turnaround is necessary, such as airports or large retail, leisure and sports venues.
The test can analyse multiple types of sample (throat and nasal swabs, in addition to saliva and sputum) and, crucially, the virus is neutralised once it is captured in a vial, making it safe for the operator administering the test. The sample is placed in a solution containing the company’s highly-sensitive magnetic beads which attract RNA from the virus, even at the lowest of levels. This allows the RNA to be extracted from the patient’s sample ready for amplification.
The detection technology is based on the biotech’s loop-mediated isothermal amplification (LAMP) technology.
MicrosensDx COVID-19 is a refined version of a prototype test which was originally designed earlier this year and validated in collaboration with Kings College London’s Professor Tim Spector OBE, the world-leading epidemiologist. The sensitivity of the test has improved markedly since the early-stage assay, with validation data due later this month to coincide with full CE marking accreditation.
Dr Mark Street-Docherty, CEO at MicrosensDx, said: “Our COVID-19 technology is the fastest test to be clinically validated. It works up to three times faster than the widely available PCR based methods, is easy to use and non-invasive.
“We’ve proved it works on a large scale over the last few months in Cumbria and are now working with our partners to ramp up production. The extraction element of our test received CE accreditation last week, with our LAMP technology due to receive its CE mark later this month. We’re now in early discussions to supply commercial operators and health authorities across Europe in the coming months.
“Rapid, accurate testing, which can also be carried out on location, is the lifeline that industries paralysed by the pandemic have been calling out for. We’re delighted to bring MicrosensDxCOVID-19 to market and look forward to collaborating with our partners to make it available to any organisation which needs it.”
In addition to detecting COVID-19, the testing platform can detect many other viral RNA targets, such as norovirus, respiratory syncytial virus (RSV) or influenza. The ability to quickly and accurately detect these other diseases has real potential to reduce inappropriate prescription of antibiotics and help to control outbreaks at their onset.
MicrosendsDx has a 20-year-long heritage in the diagnostic sector and had produced market-leading tests for BSE and tuberculosis.
Learn more - MicrosensDx COVID-19
About MicrosensDx
With almost 20 years of experience in addressing unmet needs in diagnostic markets, MicrosensDx specialises in developing cost-effective, easy to use and easily automatable technologies for the extraction and detection of intact pathogens and pathogen nucleic acids from the widest range of samples. The company has been highly successful in developing a laboratory test for the ‘mad cow’ agent BSE and with its US partner now dominates the worldwide market for prion disease testing.
In its tuberculosis (TB) detection programme, MicrosensDx is using the company’s RapiPREP® technology in products intended for patient-side testing in local clinics in developing countries where rapid disease detection is needed. The company undertakes clinical trials in South Africa and also sells simplified TB testing products in several markets worldwide, including achieving major sales in Japan. In the field of RNA virus testing, MicrosensDx already has a long running programme to develop rapid tests for a number of viral targets in collaboration with a UK hospital and so fortuitously has in-house capabilities in place to allow a rapid response to the COVID-19 pandemic.
Media Partners