Members Login

Channels
Special Offers & Promotions
Merck Millipore's New Sirtuin Assay Used to Demonstrate Novel Mechanism for SIRT1 Activation
New SIRTainty™ Class III HDAC Assay From Merck Millipore Used in Recent Study Demonstrating Novel Mechanism for Activation of SIRT1
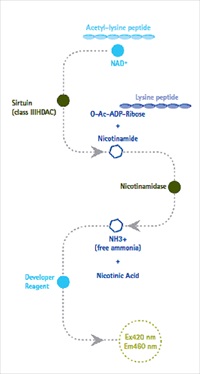 Assay format allows for simple, sensitive detection of sirtuin activity on any desired substrate
Assay format allows for simple, sensitive detection of sirtuin activity on any desired substrate - Avoids shortcomings of other sirtuin assays that require the use of specific, artificially fluorophore-tagged substrates
- Study published in Science identified novel mechanism for activation of SIRT1 using assay
Merck Millipore, the Life Science division of Merck, introduced the SIRTainty™ Class III HDAC assay which allows for simple, sensitive detection of sirtuin activity on any desired substrate. The assay utilizes a novel patent-pending technology for the sensitive detection of all known sirtuin family members. Unlike conventional assays that are dependent upon a single pre-labeled fluorescently tagged substrate, the SIRTainty™ Class III HDAC assay employs untagged acetylated peptide substrates. This approach not only enables unparalleled flexibility in the choice of sirtuin isoform and peptide substrate, but also eliminates the potential for artifacts due to the use of artificial substrates containing bulky fluorophores.
A recent report published in the March 8th issue of Science (Hubbard et al. 2013) from the lab of David Sinclair, Ph.D., Harvard Medical School, described a novel mechanism for the allosteric activation of SIRT1 by small molecules. In order to perform this work, the authors employed the SIRTainty™ assay to measure SIRT1 deacetylation of native peptide substrates.
"The SIRTainty™ assay is a superior assay for measuring sirtuin activity," said David Sinclair, Ph.D., corresponding author and co-inventor of the SIRTainty™ assay. "Unlike other HDAC assays that limit flexibility in experimental design, there is no fluorophore on the substrate. Any native substrate can be tested."
Sirtuins are a class of enzyme that removes acetyl groups from a variety of proteins that play key roles in multiple biological processes. These enzymes, also known as class III HDAC (histone deacetylases), have been implicated in a variety of age-related diseases such as cancer, Alzheimer's disease and Type 2 diabetes. Sirtuins have emerged as key targets for drug development.
"The ability to measure SIRT1 deacetylation using fluorophore-free peptides derived from physiological substrates was critical in enabling our mechanistic analysis of SIRT1 activation," noted Basil P. Hubbard, Ph.D., lead author of the article and co-inventor of the SIRTainty™ assay. "The SIRTainty™ assay is amenable to the biochemical study of multiple mammalian sirtuin deacetylases and their homologs in lower organisms. Because of this, it has the potential to facilitate the discovery and characterization of small-molecule modulators of other sirtuins."
Resveratrol, a compound found in red wine, has been shown to activate SIRT1 in vivo. However, there is controversy in the field as to whether the effect of Resveratrol on SIRT1 is direct. Previously it was shown that although Resveratrol was capable of activating SIRT1 deacetylation of a fluorescently-tagged substrate in vitro, it was unable to do so using the corresponding non-tagged peptide (1, 2). However, by using the technology now offered in the SIRTainty™ assay, the team of Harvard researchers showed that Resveratrol and synthetic SIRT1 activators can directly enhance SIRT1 deacetylation in vitro using natural amino acid substrates and SIRT1 targets containing specific hydrophobic motifs. Their work suggests that the metabolic benefits of Resveratrol are due to direct activation of SIRT1 enzymatic activity.
more about SIRTainty™ Class III HDAC assay
Media Partners


