Channels
Special Offers & Promotions
"Hand Sanitizer Analysis" - New Application Note from SCION Instruments

Introduction
Due to the corona crisis hand sanitizer became part of our daily routine. Alcohol-based hand sanitizers with at least 60 to 95% alcohol is recommended by the World Health Organisation (WHO), with the main components being ethanol, isopropyl alcohol (IPA) and/or n-propanol. To determine the effectiveness and impurities of the hand sanitizer a GC analysis can be performed. This analysis faces many challenges since hand sanitizer is made in a water matrix, which is a difficult matrix for Gas Chromatography.
International Standard Test Methods like ASTM D3695 and USP <611> covers a wide range of alcohols that can be detected and analysed quantitively and qualitatively. A GC with an FID detector is recommended for the detection of volatile alcohols in water.
Experimental
The Scion 4X6-GC analyser used for this application is equipped with an autosampler using a plunger-in-needle syringe (Both a Scion 436-and 456-GC configuration are compatible). Particular care needs to be taken when selecting injection and inlet volumes.
Backflash, a situation when solvent and sample flow back into the inlet and purge gas lines causing contamination of your system, can occur when the chosen liner volume is too small for the expansion of the water. In certain conditions 1.0 µl water can expand up to 1400 times it’s volume (1.4 ml gas). So, when choosing a liner this expansion must be considered, a 4 mm liner with glass wool is recommended. The glass wool is essential since experimentation showed that the RSD% improved in comparison with a liner without glass wool.
The focus of the calibration lies on five components that occur in hand sanitizer. ethanol, IPA and n-propanol as main components, methanol as possible contamination and glycerin which is added to the product as moisturizer. The Quality control (QC) samples are going to be prepared from 2 % (v/v) ethanol and 2,5 %(v/v) IPA.
Hand sanitizer itself usually comes in the form of a gel which is too viscous to inject, therefore the sample has to be diluted with demineralised water. An internal standard is added to the calibration, QC and hand sanitizer samples in the form of acetonitrile to compensate for the challenging matrix.
| Injector | Split/Splitless 20:1, 250ºc |
| Column | SCION-WAXMS 30m x 0.53m x 1.00um |
| Oven Program | 50°C (5 min), 30°C/min to 230°C (3 min) |
| Carrier | Helium, 7 ml/min |
| Detector | FID with ceramic jet, 250°C Air: 300 ml/min, Fuel gas (H2): 30 ml/min, Make up (N2): 23 ml/min |
| Inj. Volume | 0.2 µl |
| Autosampler | 8400 |
| Software | Compass CDS |
Table 1. Analytical Conditions
Results
The precision of the method was obtained by ten consecutive injections containing the alcohols in a range of 1-1,5 %(v/v) and internal standard. USP <611> standard prescribes a RSD% of no more than 4% for each component. Methanol showed a RSD 1.03%, IPA 1.04%, ethanol 1.19%, n-propanol 1.05% and glycerin 1.52%.
When looking at the overlay of chromatograms in figure 1 it is shown that the resolution of all components are bigger than 4 in comparison to acetonitrile (As described by USP <611>). IPA and ethanol are not baseline separated and have a resolution of 1.4, this however is sufficient for accurate quantitation.
In addition to these results USP <611> describes that the peak tailing factor (PTF) has to be smaller than 2. Methanol showed a PTF of 1.68, IPA 1.09, Ethanol 1.29, n-propanol 1.14 and glycerin 1.23.
All the components meet the requirements set by USP <611>.
Figure 1. Chromatogram overlay of the ten analyzed repeatability standards
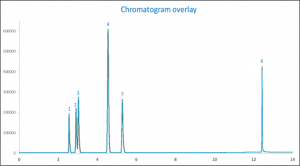
| Peak ID | Compound Name |
| 1 | Methanol |
| 2 | IPA |
| 3 | Ethanol |
| 4 | Acetonitrile (IS) |
| 5 | n-Propanol |
| 6 | Glycerin |
The calibration curves for most alcohols were prepared from 1 to 5 % (v/v). n-propanol and glycerin. n-Propanol was made from 0.1 to 1.5 % (v/v) and glycerin 0.0092 to 0.138 (v/v). The calibration curves shown in figure 2 were all made using an internal standard. The calibration range for all the alcohols except glycerin showed a correlation coefficients (R2) of at least 0.999, for glycerin this was 0.9961.
The QC sample was analysed in three runs of each 10 injections. The average concentration found for ethanol was 2.3 % (v/v) with a RSD% of 0.76, IPA was 2,5 % (v/v) with a RSD% of 1.37.
The analysis of two hand sanitizer samples was performed on a hand gel (sample 1) and a sanitizer hand rub (sample 2). According to the label of sample 1 it should contain more than 70% alcohol. The label also claims it contains glycerin and IPA but did not make any remarks about the concentration.
When analysed it showed that sample 1 contained 73.4 % (v/v) ethanol, 1.6% (v/v) IPA, 1.13 % (v/v) glycerin and 1.93 % (v/v) methanol. Figure 3 shows one of the chromatograms of sample 1.
Sample 2 contained 70 % alcohol, no further information about any other components was given. When analysed it showed that the sanitizer contained 67% (v/v) ethanol and 0.54 % (v/v) glycerin.
In order to maintain the quality of this analysis it is advised to run blanks between hand sanitizer samples and the QC samples. When the traces of the analysed components are building up it will be shown in the blanks, when this occurs clean the syringe firmly.
Figure 2. Calibration curves of the alcohols using acetonitrile as an internal standard
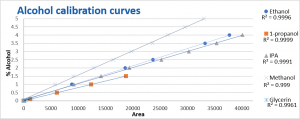
Figure 3. An example chromatogram of hand sanitizer sample 1
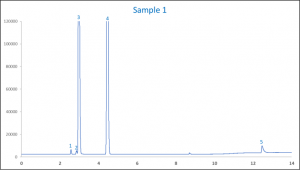
| Peak ID | Compound Name |
| 1 | Methanol |
| 2 | IPA |
| 3 | Ethanol |
| 4 | Acetonitrile (IS) |
| 5 | Glycerin |
Conclusion
The Scion 4X6-GC analyser equipped with an FID is capable of analysing hand sanitizer in a qualitative and quantitative way.
Although the developed method is fully automated and exceeding the international standard test method requirements, it is important for operators to keep an eye on the quality. Because of the challenging matrix it is important to keep a close look on the RSD% of the measurements.
When an increase in repeatability is observed, cleaning the syringe or inspecting the liner can improve the results of the analyser.

Picture 1: The Scion 4X6-GC series with 8400 autosampler
Media Partners


