Channels
Special Offers & Promotions
Avantor
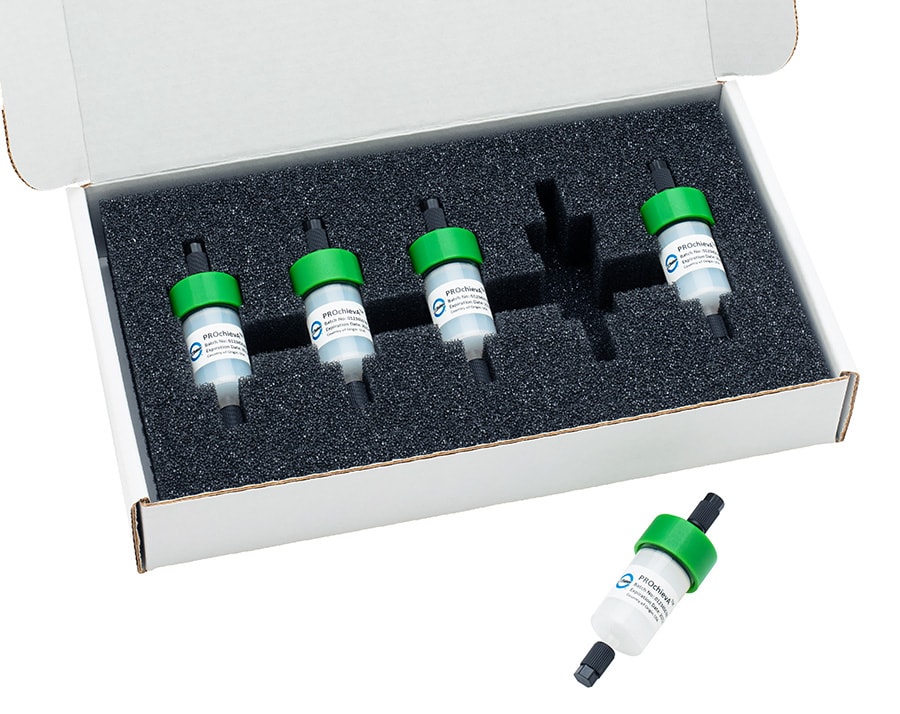
New high purity resin advances bioprocessing and brings choice and efficiencies to mAbs production workflow processes and protocols
Avantor, Inc. a leading global provider of mission-critical products and services to customers in the life sciences, advanced technologies and applied materials industries, has announced a new recombinant Protein A affinity chromatography resin used to purify antibodies during mAbs production.
Healthcare demand is increasing rapidly across most of the world, driven principally by aging populations, an increased prevalence of chronic diseases and improved access to healthcare. Monoclonal antibodies (mAbs) are proteins engineered to mimic or enhance the body's immune system. Biopharmaceutical manufacturers are pursuing ways to optimize the mAbs production process for increased efficiency, speed-to-market and cost reduction.
Protein A chromatography is a proven downstream purification step in manufacturing mAbs. Yet, there remains a need to reduce the total purification costs while improving purity and yield. The new Avantor®recombinant protein A resin addresses these challenges.
Dr. Ger Brophy, Executive Vice President for Biopharma Production at Avantor, said, "Biopharmaceutical developers and manufacturers are urgently seeking new tools to drive more efficiency in their production processes. But they cannot compromise on quality standards as they work to provide powerful medicines to patients in a timely, safe and efficient manner."
In performance tests conducted against other best-in-class Protein A resins on the market today, Avantor's novel J.T.Baker® BAKERBOND® PROchievA™ recombinant protein A resin demonstrated high performance and provided best-in-class purification in the critical Protein A affinity chromatography step of mAbs manufacturing. Avantor's resin is compatible with current manufacturing standards which ensures continuity in workflow processes and compliance protocols.
"Our resin provides customers with a best-in-class, high-performing alternative to existing purification technology, with the benefit of greater supply chain flexibility and security," added Dr. Brophy.
The BAKERBOND® PROchievA™ resin is manufactured at Avantor's Bridgewater Innovation Center in N.J., USA and is the company's latest example of driving improved bioprocessing efficiencies for the Life Sciences industry. Avantor recently teamed up with the National Institute for Bioprocessing Research and Training (NIBRT) in Dublin, Ireland to address downstream bottlenecks in buffer preparation when producing mAbs. More recently Avantor opened its ninth Innovation and Customer Support Center, which is located in Shanghai, China.
The new resin enhances Avantor's J.T.Baker® family of products for biopharmaceutical research and production. Avantor's J.T.Baker® brand chromatography products allow biopharma manufacturers to realize higher production efficiencies, meet stringent regulatory requirements and accelerate regulatory approval in bringing new therapies to market.
Visit the website for more information on BAKERBOND® PROchievA™ and about Avantor's biopharma capabilities in monoclonal antibodies, recombinant proteins, vaccines, cell and gene therapies and small molecule products.
learn more about BAKERBOND® PROchievA™ resin
learn more about Avantor's biopharma capabilities
About Avantor
Avantor® is a leading global provider of mission-critical products and services to customers in the life sciences, advanced technologies and applied materials industries. The company operates in more than 30 countries and delivers an extensive portfolio of products and services. As our channel brand, VWR offers an integrated, seamless purchasing experience that is optimized for the way our customers do business. We set science in motion to create a better world.
Media Partners