Channels
Special Offers & Promotions
OGT Launches Cytocell FISH Probe for Bladder Cancer
Cost-effective, ready-to-use kit enables appropriate testing strategies for reliable diagnoses
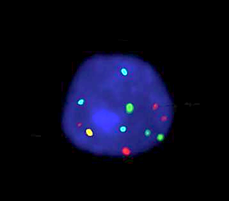 Oxford Gene Technology (OGT), The Molecular Genetics Company, has launched its CE-IVD labelled Cytocell Aquarius®P16/3c/7c/17c Probe Kit, a cost-effective, ready-to-use fluorescence in situ hybridisation (FISH) probe kit for non-invasive detection of bladder cancer — for sale in Europe.
Oxford Gene Technology (OGT), The Molecular Genetics Company, has launched its CE-IVD labelled Cytocell Aquarius®P16/3c/7c/17c Probe Kit, a cost-effective, ready-to-use fluorescence in situ hybridisation (FISH) probe kit for non-invasive detection of bladder cancer — for sale in Europe.
Bladder cancer is becoming increasingly prevalent, and in 2012 was reported as the 5th most common cancer in Europe1. While cytology screening is commonplace for diagnosing bladder cancer, a recent update to UK NICE guidelines2 recommended the use of FISH. FISH methods have been shown to have the same specificity as cytology screening and greater sensitivity in detecting bladder cancer cells3-4. FISH analysis for bladder cancer is also particularly useful for monitoring treatment outcome and can aid in reducing patient mortality by detecting bladder cancer recurrence up to six months earlier than other methods5.
Carrying the CE mark for in vitro diagnostic use within Europe, OGT’s product accurately detects in urine samples the three most common aneuploidies associated with bladder cancer (chromosomes 3, 7 and 17). It also detects deletions of the 9p21.3 locus6 containing the well-known tumour suppressor gene p16 (CDKN2A) — commonly deleted in bladder cancer7-8. The economical Cytocell Aquarius P16/3c/7c/17c Probe Kit contains ready-to-use reagents and has an easy-to-use protocol, reducing the potential for errors and increasing convenience. Specific, clear, high-intensity signals with minimal background deliver quality, reproducible and easy-to-score results ensuring clinicians can be confident in reporting.
Dr. Lorenza Pecciarini, cytogeneticist at the Saint Raphael Hospital in Milan, who extensively tested the probe on a large series of samples, commented: “We found Cytocell’s bladder cancer probe kit easy to use, with bright results that were simple to interpret. It was straightforward to incorporate into our existing cytology workflow and was highly reproducible. This product offers an accurate and cost-effective technology for the diagnosis of bladder cancer.”
Martin Lawrie, Managing Director at Cytocell said: “The decision to expand OGT’s cancer portfolio and specifically Cytocell pathology probes, demonstrates OGT’s commitment to the fight against cancer. Supported by recent guidelines, our new bladder cancer probe kit delivers a convenient means to confident diagnoses, backed by the great customer support that OGT has become renowned for.”
more about oxford gene technology
more news from oxford gene technology
Media Partners


