Channels
Special Offers & Promotions
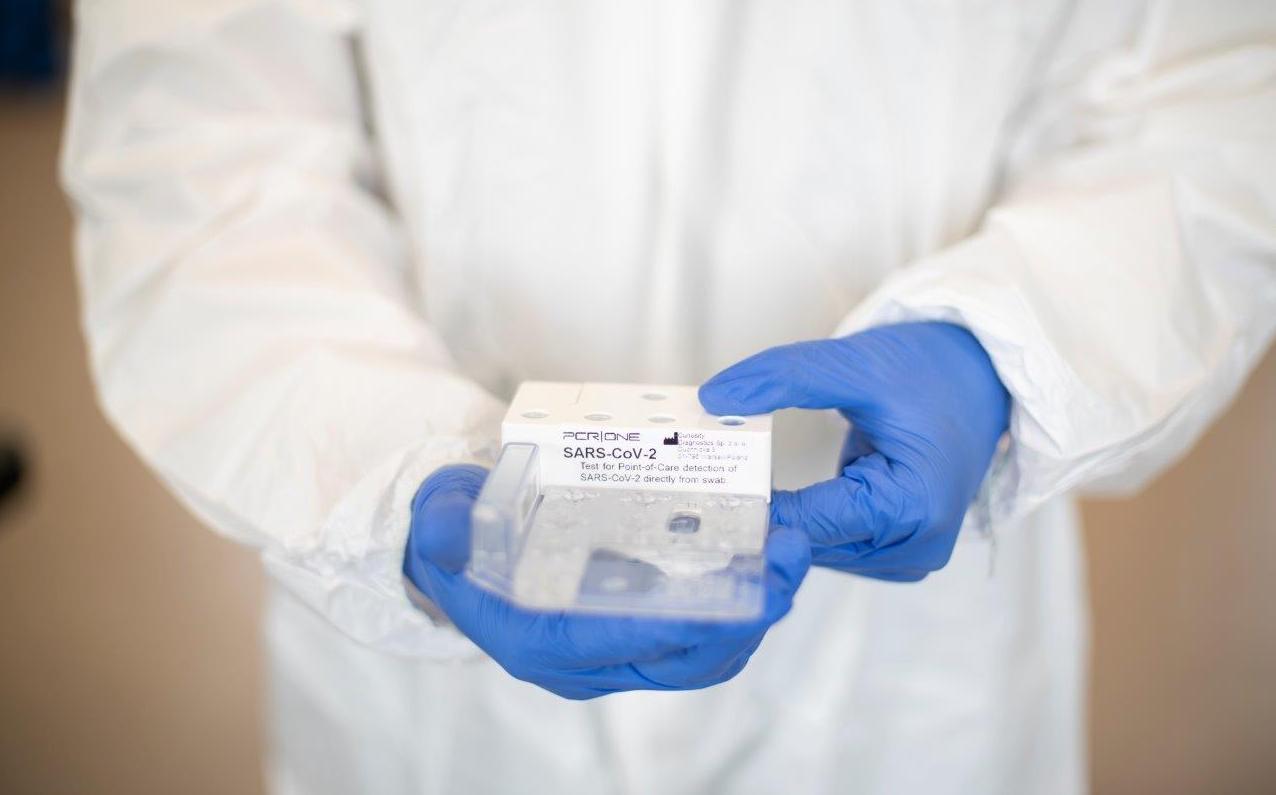
Scope Fluidics launches clinical tests of the PCR|ONE SARS-Cov-2 panel in the clinical environment
Scope Fluidics – a biotechnological company listed on NewConnect, developer of innovative medical diagnostics projects – has commenced prevalidation tests of the PCR|ONE SARS-CoV-2 panel on clinical samples.
The tests will be conducted in a hospital laboratory, on swabs collected directly from patients. The aim of the initiated tests is to check the effectiveness of the developed panel, which has so far been validated in the course of internal tests. According to the schedule, certification of the PCR|ONE SARS-CoV-2 panel is to take place in the Q4 2020.
The purpose of performing prevalidation tests of the PCR|ONE SARS-CoV-2 panel in an external laboratory is to assess the panel’s performance on samples/swabs taken from patients compared to the diagnostic systems that the hospital is currently using on a regular basis. The project received a positive opinion of the Bioethics Commission.
"Within few months we have developed a fully automatic, genetic method for detecting SARS-CoV-2 coronavirus directly from nasal swabs, at the place of sampling. Now, in the last phase of preparation for formal certification tests, we are verifying the test's performance on clinical samples. Direct contact with the clinical environment has a special significance. Access to rapid and reliable diagnostics greatly facilitates hospital logistics and increases patients' comfort – we all know how expensive and difficult it is to isolate patients, both for them and their families", says prof. Piotr Garstecki, co-founder, key shareholder and CEO of Scope Fluidics.
In August this year, Scope Fluidics announced the commencement of preparations for the accelerated registration procedure of the PCR|ONE SARS-CoV-2 panel in the USA, as a part of the FDA EUA (Emergency Use Authorization) procedure. EUA is an accelerated procedure of releasing a medical product onto the US market by the U.S. Food and Drug Administration (FDA). It is applied in emergency situations, when there is a threat to public health. The choice of a referencing method, collecting clinical samples and the analysis of the results will be conducted in compliance with the FDA recommendations and relevant ethical, legal and scientific regulations and standards. The company has also announced its plans to obtain the European market certification (CE-IVD) in Q4 2020.
Scope Fluidics, having obtained a CE-IVD certificate for the device, plans to carry out from several to several dozen thousands of tests in health care centres and other public and private institutions across Poland and Europe.
The market of Point-of-Care molecular diagnostics, which has been developing rapidly over the last several years, has increased several times in the period of the pandemic. It is predicted that the increased demand for Point-of-Care genetic tests will persist for the next couple of years.
Scope Fluidics SA is a hub that grows med-tech start-ups, each developing a radical solution to a challenge in global health. The company responds to the global challenges in human health, by designing disruptive diagnostic systems, with the potential to positively impact access to effective healthcare across the world. PCR|ONE system targets healthcare-associated infections and infection control, by offering unprecedented access to molecular Dx information at the point of care. BacterOMIC promises access to precision antibiotic therapies at the price of conventional antibiotic susceptibility testing.
PCR|ONE system is a complete, stand-alone solution for immediate detection of a wide range of pathogens and genetic targets in the point of care format. The system accepts disposable cartridges that are loaded with clinical samples and automatically processed for detailed diagnosis within 15 minutes. The PCR|ONE system offers a unique combination of record speed and breadth of up to 20 genetic targets that can be identified in a single run, from a single sample.
BacterOMIC is the first system to offer full and actionable information for precision antibiotics therapies. BacterOMIC problems all clinically relevant antibiotics in single test. BacterOMIC
is also the first system to offer automated testing of multiple antibiotic combinations.
Media Partners