Members Login

Channels
Special Offers & Promotions
OGT Introduces Unique CNV Array with Whole Chromosome Uniparental Disomy (UPD) Detection
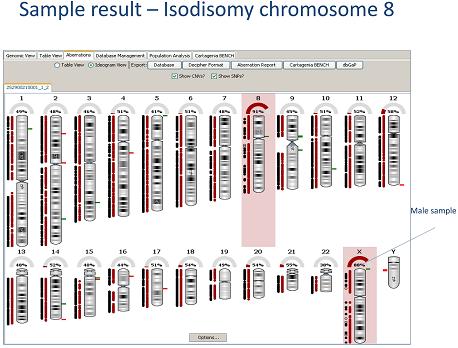 Oxford Gene Technology (OGT), provider of innovative clinical genetics and diagnostic solutions to advance molecular medicine, has today introduced the groundbreaking CytoSureTM ISCA UPD 4 x 180k array. This offers the ability, for the first time, to simultaneously detect DNA copy number variation (CNV) using OGT's ISCA consortium endorsed 4 x 180k aCGH array, along with whole chromosome uniparental disomy (UPD) using SNP probes, on a single array. The array format, ease-of-use and intuitive data interpretation via the complimentary CytoSure Interpret Software, combine to make the CytoSure ISCA UPD array an obvious choice for the next stage in cytogenetic research.
Oxford Gene Technology (OGT), provider of innovative clinical genetics and diagnostic solutions to advance molecular medicine, has today introduced the groundbreaking CytoSureTM ISCA UPD 4 x 180k array. This offers the ability, for the first time, to simultaneously detect DNA copy number variation (CNV) using OGT's ISCA consortium endorsed 4 x 180k aCGH array, along with whole chromosome uniparental disomy (UPD) using SNP probes, on a single array. The array format, ease-of-use and intuitive data interpretation via the complimentary CytoSure Interpret Software, combine to make the CytoSure ISCA UPD array an obvious choice for the next stage in cytogenetic research.
OGT's unique UPD detection capability (patent pending) has been enabled by multiple rounds of SNP probe selection and validation, targeting over 6000 SNPs with evenly distributed probes, resulting in highly informative content that allows confident detection of whole chromosome UPD. Furthermore, the combined ISCA-UPD array has been developed to ensure near identical labelling and hybridisation conditions to standard aCGH. In addition, the aCGH protocol is largely unaltered and any reference DNA can be used. The latest version of OGT's CytoSure Interpret Software provides simple and intuitive data analysis, with clear identification of regions with a loss of heterozygosity (LOH) and data processing tools to investigate these further. As a result of this unique array design and data analysis capability, segmental as well as whole chromosome UPD can be detected using the CytoSure ISCA UPD array.
James Clough, Vice President Clinical and Genomic Solutions at OGT commented: "We have carefully implemented robust UPD detection capabilities onto our CytoSure ISCA 4 x 180k arrays, whilst avoiding wholesale changes to the protocol. Importantly, we have also made the interpretation of this additional data extremely intuitive via our CytoSure Interpret Software. As a result, our customers can now confidently detect both DNA copy number variation and whole chromosomal uniparental disomy using a single array."
OGT's powerful CytoSure ISCA arrays have been carefully developed to focus on disease and syndrome-associated genome regions, in addition to offering whole genome coverage. Using a proprietary 60-mer probe design and multiple rounds of optimisation, the CytoSure ISCA aCGH arrays ensure exceptional reliability and confident detection of genetic aberrations with high signal-to-noise ratios.
For further information on OGT's cytogenetics products and services please visit www.ogt.co.uk.
Media Partners