Channels
Special Offers & Promotions
YMC-Triart Prep - Application Note: Separation of structurally similar peptides at pH ? 7
Reversed phase chromatography is undoubtedly the most powerful method for peptide purification. In order to improve the cost effectiveness of industrial scale peptide purifications, YMC has developed a new hybrid silica base and a novel bonding technology to improve process efficiencies and inrease column life time.
The new hybrid silica base, named YMC-Triart, was successfully prepared using micro-reaction flow technology. This allows the production of completely spherical, porous beads with a layered hybrid structure.
Compared to conventional silica based materials, the new hybrid particles show an enhanced stability in alkaline pH mobile phases, allowing a wider flexibility in separation conditions over an extended pH range. In addition, the new hybrid silica gels can be cleaned in place (CIP) with NaOH-containing buffers. This improves packed column life-time in peptide purification processes.
Separation of angiotensins
Human angiotensin II and III contain the same seven amino acid sequence. Angiotensin II differs from angiotensin III only by an additional N-terminal aspartate.
Structurally similar peptides such as angiotensin II and III can be difficult to separate under the low pH conditions that are typically used for peptide separations. With the increased pH stability of YMC-Triart, resolution can be increased by using neutral to alkaline pH conditions.
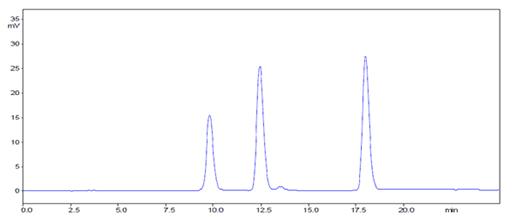
Column | : Triart C8-L (20 nm, 10 µm), 250 x 4.6 mmID |
YMC-Triart C8-L is available in prepacked columns and in bulk.
If you are interested in more information or if you would like to test a scout column or a sample please contact Dr. Britta Blödorn, YMC Europe GmbH, Phone: +49 (0)2064 427-271, Fax: +49 (0)2064 427-222
or visit www.ymc.de
Media Partners


