Channels
Special Offers & Promotions
Smith & Nephew Announce European Launch of Handheld Imaging Technology Heralding a New Era of Evidence-Based Decision Making in Wound Care
Smith & Nephew, the global medical technology business, announces the European launch of MolecuLight i:XTM, the easy to use, handheld imaging device that instantly measures wound surface area and visualises the presence and distribution of potentially harmful bacteria in wounds.
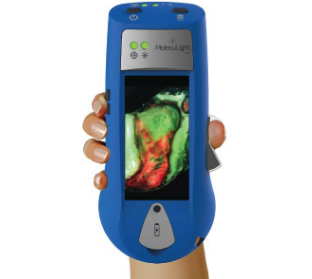 Currently wound assessments are made with the naked eye which can lack the accuracy required to most effectively guide clinical decision making. Using fluorescence, MolecuLight i:X quickly, safely, and easily visualises potentially harmful bacteria in wounds which may otherwise lack signs or symptoms of infection. It enhances a clinician’s ability to choose the right therapy, at the right time for their patient and can help to guide wound sampling and debridement, monitor wound progression, improve patient engagement and simplify wound documentation.
Currently wound assessments are made with the naked eye which can lack the accuracy required to most effectively guide clinical decision making. Using fluorescence, MolecuLight i:X quickly, safely, and easily visualises potentially harmful bacteria in wounds which may otherwise lack signs or symptoms of infection. It enhances a clinician’s ability to choose the right therapy, at the right time for their patient and can help to guide wound sampling and debridement, monitor wound progression, improve patient engagement and simplify wound documentation.
Clinical data from wound assessments demonstrates that incorporating the MolecuLight i:X into standard care facilitated more objective medical decision making and led to up to 9 times faster wound healing and 54% more accurate swabbing.
“The MolecuLight i:X enables healthcare professionals to see what they have never been able to see before“ said Paolo Di Vincenzo, Smith & Nephew’s Senior Vice President Global Marketing, Wound.“We are proud to partner with MolecuLight Inc. and bring this innovative technology to our customers. It strongly complements our range of advanced wound care products and we believe is set to start a revolution in wound care clinical practice.”
“For the first time clinicians can accurately sample a wound in situ to determine if bacteria are present as well as more effectively debride a wound under fluorescence visualisation. These are fundamental areas of everyday wound care that have remained suboptimal for too long, until now,” says Dr. Ralph DaCosta, Founder, Chief Scientific Officer and Director, MolecuLight Inc.
An estimated 2 million people are living with a chronic wound across Europe and an estimated 16% of all chronic wounds remain unresolved after a year or longer. Ensuring wounds are appropriately diagnosed and treated is a priority for healthcare providers across Europe, reducing cost and improving outcomes for patients.
“Not only has the MolecuLight i:X transformed my department’s clinical decision making in terms of targeting sampling and debridement and improving antimicrobial stewardship, but the benefit to patients has also been exciting to see,” says Rosemary Hill*, Canadian Wound Ostomy Continence Nurse Clinician, Lions Gate Hospital, Vancouver. “By being able to engage patients in their wound healing progress, and by showing them the real-time images, we can reduce anxiety, and provide reassurance regarding the diminishing burden of bacteria.”
The MolecuLight i:X Imaging Device is approved by Health Canada (Medical License #95784) and has CE Marking (Certificate # G1160292355002) for sale in the European Union. The MolecuLight i:X Imaging Device is not available in the US.
Media Partners


