Channels
Special Offers & Promotions
NEW eAST Automated Zone Measurement Software Upgrade
Accurately Generates and Analyses Data for Clinical Use and Antibiotic Research
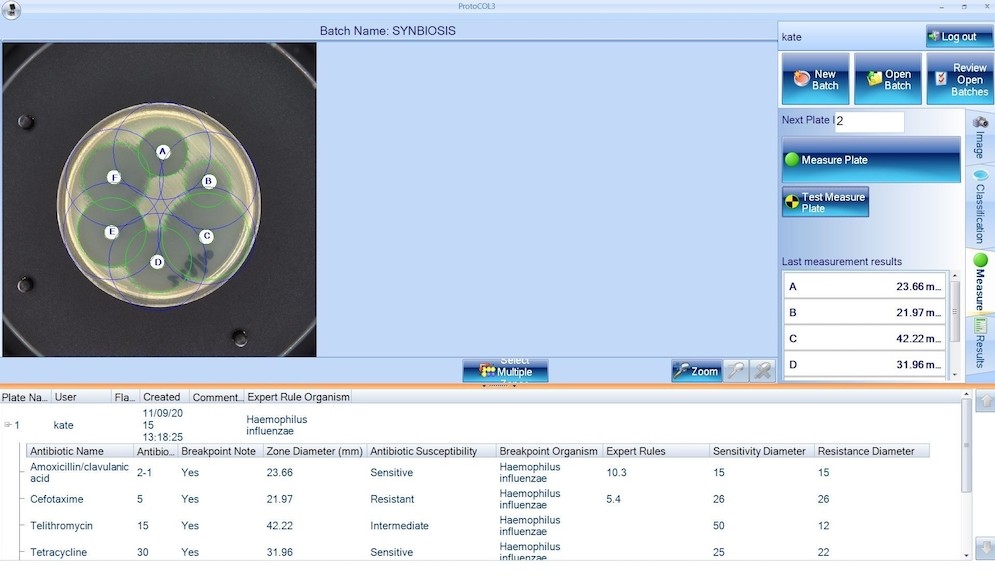
Synbiosis, a long-established, expert manufacturer of automated microbiological systems, is delighted to announce its eAST software for automatic zone measurement of antimicrobial susceptibility testing (AST) plates has been upgraded to improve antibiotic SIR (Susceptible, Intermediate, Resistant) category determination. The new software rapidly generates and analyses AST data, making it ideal for use in regulated clinical and antibiotic development laboratories.
The new eAST software now has all the 2018 breakpoint values for European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical Laboratory Standards Institute (CLSI) guidelines included in its expert rules database. Using the software, microbiologists can in less than half the time it would take to analyse breakpoint results manually, automatically compare zone measurements to current breakpoint values and obtain a list of SIR categories, as well as determine antibiotic efficacy and produce guidelines for treatment options.
The plate images can be stored in a secure SQL database for cross reference and numerical data can be transferred to a spreadsheet (Excel/ OpenOffice) or LIMS. This eliminates keying and data transfer errors, producing fully traceable results, which are consistent from microbiologist to microbiologist.
The software is GLP and GDP compliant and can be used in a 21 CFR Part 11 environment because it has user access levels and a full audit trail with user login in and logout records. These archived results are suitable for generating reports for audit by regulatory authorities and can also be used in hospitals wanting to identify and monitor incidence of microbial resistance, making the new eAST software suitable for use in highly regulated microbiology laboratories.
The eAST software is included in the IVD-registered ChromaZona as standard and is available as an optional module for ProtoCOL 3 systems. | find out more about the new eAST software upgrade. Existing eAST software users can download the upgrade free of charge
“EUCAST and CLSI breakpoint values are the most popular worldwide guidelines for AST work but manually analysing the data is error prone and subjective,” explains Kate George, Sales and Technical Director at Synbiosis, “Adding the most up-to-date values to upgrade the software provides an excellent solution to this problem. We’re confident that microbiologists using our new eAST software can both rapidly and accurately determine the efficacy of their antibiotics or diagnose which antibiotic to use to treat serious microbial infections.”
About Synbiosis
Synbiosis is a world-leading supplier of integrated imaging solutions for automatic counting and analysis of microbial colonies and zone measurement. The ProtoCOL, Protos and aCOLyte systems from Synbiosis are installed in food, pharmaceutical, environmental and research microbiology laboratories world-wide. ChromaZona is an IVD certified instrument for automated microbial ID and AST in the clinical laboratory. Synbiosis uses established distribution channels to market its products internationally.
Synbiosis, founded in 1998 is a division of the Synoptics Group of the AIM quoted Scientific Digital Imaging Company based in Cambridge, UK. The Group’s other divisions, Syngene and Synoptics Health, specialise in digital imaging solutions for molecular biology and healthcare applications respectively. Synoptics, which celebrated its 30th anniversary of being in business in 2015, currently employs 40 people in its UK and subsidiary operation in Frederick, USA.
Media Partners