Channels
Special Offers & Promotions
SyntheticMR Receives Regulatory Approval for SyMRI 15 in Switzerland
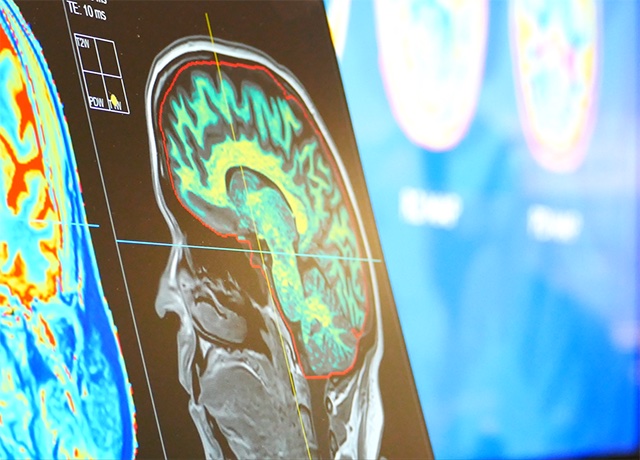
The future of MRI
SyntheticMR AB, global leader in advanced quantitative imaging software, is pleased to announce that its SyMRI 15 solution has received regulatory clearance in Switzerland. This milestone enables the company to market and distribute SyMRI 15 across the Swiss healthcare sector, marking a significant stride in SyntheticMR's strategic growth within Europe.
SyMRI 15 is a groundbreaking imaging solution that revolutionizes conventional magnetic resonance imaging (MRI) practices. Clinically validated through an extensive multi-center study involving several top-tier institutes in the United States, SyMRI 15 has demonstrated the capability to replace traditional 3D imaging techniques. The study highlighted significant enhancements in efficiency and throughput, without compromising diagnostic accuracy.
"Entering the Swiss market is a pivotal moment for SyntheticMR," said Ulrik Harrysson, CEO of SyntheticMR AB. "Switzerland is renowned for its high standards in healthcare, and the approval of SyMRI 15 here underscores the quality and efficacy of our technology. We are committed to partnering with Swiss healthcare providers to transform medical diagnostics and improve patient outcomes."
About SyMRI
SyMRI provides multiple contrast-weighted images and quantitative information about the patient in a single fast scan, enabling users to speed up their imaging workflow and add objective decision support to their practice.
About SyntheticMR
SyntheticMR AB develops and markets innovative software solutions for Magnetic Resonance Imaging (MRI). SyntheticMR
AB has developed SyMRI®, delivering multiple, adjustable contrast images and quantitative data from a single 5-minute scan. The SyMRI product is available in different packages. SyMRI NEURO delivers multiple contrast images, tissue segmentations and quantitative data on the brain. SyMRI MSK provides multiple contrast images and quantitative data for knee and spine anatomies. SyMRI NEURO is CE-marked, and FDA 510(k) cleared and SyMRI MSK is CE-marked. SyMRI 3D is 510(k)-pending and CE-marked. SyMRI is a registered trademark in Europe and the USA. SyntheticMR is listed on the Spotlight Stock Market Exchange in Stockholm, Sweden.
Media Partners


