Channels
Special Offers & Promotions
Scientists tackle difficult-to-recycle thermoset polymers

A team of UK scientists has got a step closer to making several different types of plastic much easier to recycle, using a method that could be applied to a whole range of difficult-to-recycle polymers, including rubbers, gels and adhesives.
Thermoplastics and thermosets are two types of plastics that both consist of long chains of molecules called polymers but behave differently when heated.
Thermoplastics can be heated to high temperatures, poured into a mould then cooled to make the desired shape. They can subsequently be melted and reformed into other shapes when they are recycled, however they can break when stretched or stressed.
In contrast, the polymer chains in thermoset plastics are crosslinked to form a network which makes them incredibly strong and flexible. They are often used in composite materials, paints, coatings, rubbers and gels. Unfortunately, however, the crosslinks mean that the materials burn rather than melt when heated, making them much harder to break down and recycle.
Now, researchers at the University of Bath and University of Surrey have developed a way of introducing degradable bonds into thermoset polymers to make them more easily recyclable.
Publishing in Polymer Chemistry, they made a series of polymer gels with breakable bonds incorporated into different parts of the structure, and tested whether the properties changed after the gel was degraded and reformed.
They found that whilst all the gels could be degraded to some extent, gels with breakable bonds in the polymer chains (B in the diagram below) retained their properties much better when reformed, compared with the polymers that were broken down via the cross-linked bonds (A).
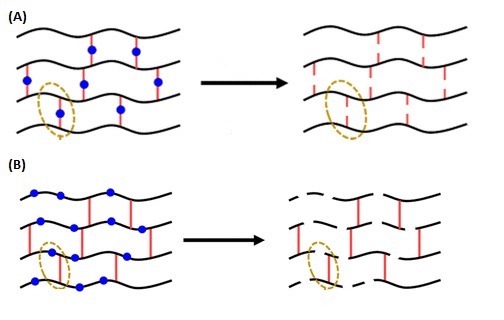
The researchers hope this model system can be applied to other types of polymers, including adhesives, sealants and elastomers.
Dr Maciek Kopeć, from the University of Bath’s Department of Chemistry, said: “Thermosets are used widely in the commercial sector, in materials like resins and adhesives.
“Being able to make bonds reversible in these materials will increase their applications as well as making them more recyclable.”
The researchers aim to create a general road map of the best locations for these breakable bonds, to understand better why some bonds break more easily than others, and plan to optimise the system using other commercially used polymers.
The researchers are also looking at other applications of the work, including using crosslinked polymers as vehicles for controlled drug delivery systems.
The work was funded by the Engineering and Physical Sciences Research Council (EPSRC).
The University of Bath is one of the UK's leading universities for high-impact research with a reputation for excellence in education, student experience and graduate prospects.
We are named ‘University of the Year’ in The Times and The Sunday Times Good University Guide 2023, and ranked among the world’s top 10% of universities, placing 148th in the QS World University Rankings 2024. We are ranked 5th in the UK in the Complete University Guide 2024, 6th in the Guardian University Guide 2024 and 8th in the The Times and Sunday Times Good University Guide 2024.
Bath is rated in the world’s top 10 universities for sport in the QS World University Ranking by Subject 2023. We produce some of the world’s most job-ready graduates and were named University of the Year for Graduate Jobs by the Daily Mail University Guide 2024, as well as ranking as one of the world’s top 90 universities for employer reputation according to the QS World University Rankings 2024.
Research from Bath is helping to change the world for the better. Across the University’s three Faculties and School of Management, our research is making an impact in society, leading to low-carbon living, positive digital futures, and improved health and wellbeing.
Media Partners


