Channels
Special Offers & Promotions
Indica Labs digital pathology platform chosen by the Institute of Tissue Medicine to transform the way pathologists and researchers work
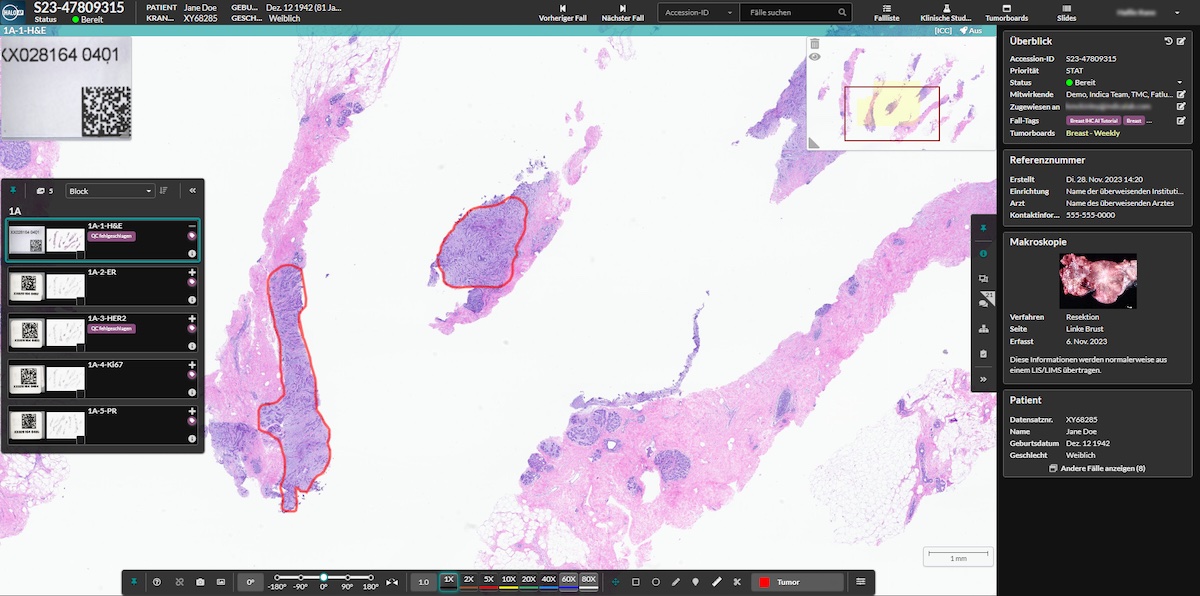
The Institute of Tissue Medicine of the University of Bern Chooses Indica Labs’ HALO AP® to Become the First Public Health Institute in Switzerland to Deploy Digital Pathology for Diagnostics in the Cloud with Amazon Web Services (AWS)
Indica Labs, the global leader in AI-powered digital pathology, announces that the Institute of Tissue Medicine and Pathology at the University of Bern, a leading university based pathology institute in Switzerland, has selected HALO AP® for the Institute’s first-in-country digital pathology deployment in the cloud powered by Amazon Web Services, Inc. (AWS). The Institute selected Indica Labs for its ability to provide dependable, pathologist-centered software and AI in a powerful and highly flexible platform.
HALO AP®, an enterprise digital pathology platform from Indica Labs, transforms the way pathologists and researchers work. To address the complex needs of modern pathology laboratories, HALO AP® features a comprehensive suite of tools and workflows for efficient and accurate image analysis, diagnostics, and collaboration. The platform is highly interoperable and works with most scanners and file formats. Users can adapt their deployment to meet their needs with both cloud and on-premise deployment options and are free to incorporate their existing laboratory equipment with ease, allowing them to customize their environment for the practice of pathology.
With the professional consulting support of AWS partner foresite AG (www.foresite.ch) for requirements analysis as well as the creation, submission and follow-up of all documents for the Data Protection Officer, the Institute has achieved a significant milestone by being the first public health institution in Switzerland to receive local authority approval to utilize AWS for the public cloud management of personal healthcare data in diagnostics. This first-in-country approval by Bern Canton’s data privacy office allows the Institute to store encrypted patient data in AWS Zurich region. Cloud technology is highly scalable, cost-effective, and has built-in security and flexibility which is particularly well-suited for evolving needs in digital pathology. To facilitate a smooth implementation of and transition to the new digital pathology platform, the Institute will be working closely with the Cloud Services team from Indica Labs. This collaborative effort will optimize the integration of the new platform with the Institute's existing systems and workflows, maximizing the benefit physicians and patients experience from the cloud-based solution.
"The implementation of HALO AP for digital tissue medicine at the University of Bern represents a major step towards streamlining diagnostic workflows and preparing for a future with AI-enabled pathology," said Prof. Dr. Aurel Perren of the Institute of Tissue Medicine and Pathology at the University of Bern. "We anticipate that deploying digital pathology in a secure AWS environment tailored to our institute’s needs will positively impact turnaround time, diagnostic quality and will ultimately contribute to improving patient care."
"We are thrilled to work with the Institute of Tissue Medicine and Pathology at the University of Bern," said Eric Runde, COO of Indica Labs. "This partnership will create a truly bench-to-bedside approach to pathology that will improve patient care and diagnostic efficiency. By utilizing our Cloud Services team for this deployment, the Institute will have a truly scalable digital pathology solution with the support they need to run an efficient and patient-centered service."
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP® is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP® provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.
About Indica Labs
Indica Labs is the global leader in AI-powered digital pathology software and services. Our flagship HALO® and HALO AI platform revolutionizes quantitative evaluation of whole slide images. HALO Link provides collaborative image management while HALO AP® and HALO AP Dx deliver enterprise digital pathology for primary diagnosis with regulatory clearances in multiple markets. Through a commitment to open pathology, performance, scalability, and ease-of-use, we help pharma companies, diagnostic labs, hospitals, research organizations, and Indica’s own Cloud and Pharma Services make discoveries and diagnoses that transform patient care and scientific discovery.
Media Partners


