Channels
Special Offers & Promotions
FDA Issue Emergency Use Authorization to Thermo Fisher Scientific for Diagnostic Test Used to Detect COVID-19
Thermo Fisher Scientific Inc., the world leader in serving science, announced that on March 13, 2020, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for its diagnostic test that can be used immediately by CLIA high-complexity laboratories in the U.S. to detect nucleic acid from SARS-CoV-2, the virus that causes COVID-19, and not for any other viruses or pathogens.
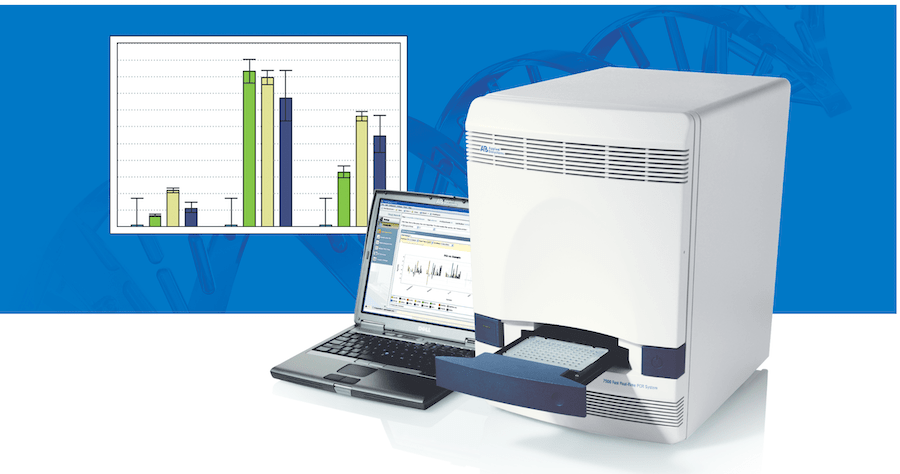
The authorized test uses Applied Biosystems TaqPath Assay technology and is designed to provide patient results within four hours of a sample being received by a lab. The estimated time-to-result also includes time for sample preparation and instrument analysis.
On March 13, Marc N. Casper, chairman, president and chief executive officer of Thermo Fisher Scientific, said, "The authorization of our diagnostic test for COVID-19 will help to protect patients and enable medical staff to respond swiftly to treat those who are ill and prevent the spread of infection. At Thermo Fisher, our Mission is to enable our customers to make the world healthier, cleaner and safer. In partnership with the FDA and regulatory authorities around the world, we are committed to expanding the availability of diagnostic testing to prevent the spread of this disease."
Today Thermo Fisher also provided an update on its anticipated production rate. The company currently has 1.5 million tests available to ship under the EUA label and expects to quickly ramp up to reach 2 million tests per week. Based on availability of raw materials and an installed instrument base, the company expects to scale production up to 5 million tests per week during the month of April. The available tests will initially be distributed to approximately 200 labs in the U.S. and Thermo Fisher will continue to work in partnership with government agencies and private partners to expand access.
The EUA test is optimized for use on the company's Applied Biosystems 7500 Fast Dx Real-time PCR instrument, which is covered under the EUA and already used in clinical laboratories worldwide.
This test has not been FDA cleared or approved, however, the FDA can issue an EUA to permit use of certain medical products that may be effective in diagnosing, treating or preventing a disease or condition, as in the case of the novel coronavirus when the secretary of the U.S. Department of Health and Human Services (HHS) declares a public health emergency. HHS Secretary Alex Azar declared an emergency for COVID-19 on January 31. The test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
About Thermo Fisher Scientific
Thermo Fisher Scientific Inc. is the world leader in serving science, with annual revenue exceeding $25 billion. Our Mission is to enable our customers to make the world healthier, cleaner and safer. Whether our customers are accelerating life sciences research, solving complex analytical challenges, improving patient diagnostics and therapies or increasing productivity in their laboratories, we are here to support them. Our global team of more than 75,000 colleagues delivers an unrivaled combination of innovative technologies, purchasing convenience and pharmaceutical services through our industry-leading brands, including Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific, Unity Lab Services and Patheon.
Media Partners