Channels
Special Offers & Promotions
EKF launches PrimeStore
New sample collection kit enables convenient and safe collection, transportation and handling of pathogenic samples including COVID-19 and influenza
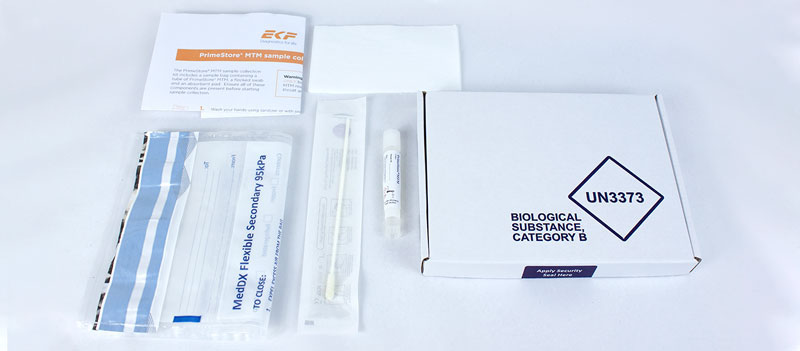
EKF Diagnostics, the global in vitro diagnostics company, announces that it has launched a PrimeStore® MTM sample collection kit. This new kit enables convenient and safe collection, transportation and handling of pathogenic samples, including COVID-19 and influenza. Two kit configurations are now available: a postal sample kit ideal for home use and a multi-pack of sample kits suitable for on-site mass sampling. To meet growing demand for this sample collection device, EKF has increased its manufacturing capacity in the UK and mainland Europe.
Pathogenic samples stored in the FDA-cleared, CE-IVD marked PrimeStore MTM novel viral transport media, including SARS-CoV-2, are inactivated within minutes of collection. This allows such samples to be transported safely through the postal system or via courier and complies with UN3373 packaging regulations. Furthermore, as samples are stable in PrimeStore MTM for seven days at ambient temperatures, this removes the need for all cold chain logistics during sample collection, transportation and storage. Thereby, preventing mishandled samples and making postal collection a realistic option.
Multiple nucleic acid species can be tested from one sample as RNA and DNA are perfectly preserved and stabilised by PrimeStore MTM for downstream molecular applications. Furthermore, because PrimeStore MTM totally inactivates SARS-CoV-2, as demonstrated by a recent Public Health England evaluation study, the risk of infection to lab staff from samples is eliminated. This means that samples are not only safe during transportation, but also ready for immediate safe testing at a laboratory with no need for containment.
The new sample collection kit pairs PrimeStore molecular transport media in a cryovial with a sterile flocked swab (for nasal and oral sampling) in a CE marked kit, which also includes a UN3373 95kPa compliant specimen collection bag, absorbent pad and instructions for use. In addition, the postal sample collection kit includes a UN3373 compliant postal box with security seal for sample return and postal pouch for collection kit delivery.
Due to the current COVID-19 pandemic, EKF has received a number of new orders for PrimeStore MTM sample collection devices; including initial orders from two new customers, the Health Service Executive (HSE), Ireland and a national health body in England. These add to the growing demand for PrimeStore from healthcare, education and industry to supply safe sample collection kits for COVID-19 testing programmes.
To ensure that it can fulfil the increasing orders for PrimeStore MTM and the new sample collection kit, EKF is ramping up manufacture in the UK and also mainland Europe. The Company has now started production at its facilities in Barleben, Germany, and will soon be moving its current Penarth-based manufacturing lines in Wales to a new larger facility in Llandough, Cardiff, which is over 600 sq. metres in size. This represents a 100% increase in manufacturing area.
PrimeStore® MTM sample collection kit
About EKF Diagnostics
EKF Diagnostics Holdings plc, which includes the EKF Diagnostics, Stanbio Laboratory and DiaSpect brands, specializes in the development, production and worldwide distribution of point-of-care analyzers for use in the detection and management of diabetes and anemia, and also in sports and maternal medicine. EKF products are sold in more than 100 countries around the globe, through a network of specialist distributors.
Point-of-care diagnostics: EKF Diagnostics designs and manufactures world-class diagnostic devices, as well as distributing rapid test kits for infectious diseases and pregnancy. The EKF analyzer range is used widely in GP surgeries, pharmacies, blood banks, sports clinics, hospitals and laboratories for glucose, lactate, hemoglobin, hematocrit and HbA1c measurement.
Central laboratory: EKF, through its wholly owned subsidiary, Stanbio Laboratory (Boerne, Texas, USA), manufactures a comprehensive range of clinical chemistry reagents, as well as associated analyzers. In addition, EKF Life Sciences (Elkhart, Indiana, USA) manufactures enzymes used in reagent development and also provides contract fermentation facilities.
Media Partners