Members Login

Channels
Special Offers & Promotions
Comminution of Pills - New App Note from Fritsch
The determination of substances contained in tablets after the production process is mandated according to the analytical rules of the German and European Pharmacopoeia. These rules include an analysis in regards to quality, effectiveness and safety of all contained active components and auxiliary materials.
The importance of Comminution
 The process steps are divided into comminution, dissolution and ensuing analysis. In the analytical pharmacopoeia are besides the direct titration, especially the chromatographic methods such as ion chromatography, HPLC and gas chromatography utilized. A prerequisite for these technologies is that the samples have to be in liquid and filtrated form before they can be added onto the column. The dissolution of the tablets proves to be very difficult with certain active components. Therefore it is during the comminution process extremely important to keep the particle size possibly small or obtain a suspension by a wet grinding, which can be diluted even further.
The process steps are divided into comminution, dissolution and ensuing analysis. In the analytical pharmacopoeia are besides the direct titration, especially the chromatographic methods such as ion chromatography, HPLC and gas chromatography utilized. A prerequisite for these technologies is that the samples have to be in liquid and filtrated form before they can be added onto the column. The dissolution of the tablets proves to be very difficult with certain active components. Therefore it is during the comminution process extremely important to keep the particle size possibly small or obtain a suspension by a wet grinding, which can be diluted even further.
Classical methods of the comminution with the mortar mill offer the advantage of a gentle comminution i.e. the thermal load of the sample is kept at a minimum. This comminution is utilized with especially sensitive analytes in order not to destroy these during sample preparation. But the obtainable final fineness is limited and the ensuing dissolution afterwards can also be a problem. The innovative manner of the comminution with the Planetary Micro Mill PULVERISETTE 7 premium line obtains within brief periods particle sizes below the critical area even with problematic compositions of active ingredients. The dissolution already begins during the wet grinding process, so the obtained suspension can be diluted unproblematically for the ensuing analysis.
Introduction
The production of medication is subject to very stringent guidelines and must therefore in regards to the product quality meet highest standards. During the production of the various active and auxiliary components each process step is precisely monitored. In order to guarantee an accurate analysis the repetition of mistakes must be stopped directly in the first process chain. Here the first step is always the sample preparation. In the analysis of tablets it is specifically the comminution. The further success of the analysis also depends directly on the selection of the method and the parameters to be adjusted. [1,8]
Classical vs. Innovative Methods
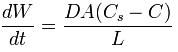
The comminution process belongs to the basic operations in pharmacy. Since humans produced their first phytopharmaceuticals themselves, they realized that the extraction of an active component is more optimal in finer substances. Dissolving depends on the interplay of solvent particles and the particles which the substances form.
The dissolving occurs on the surface of the substance. The larger the surface of a certain amount of a substance is, the faster it is dissolved. Therewith the relationship between particle size for example of a powder and its dissolving ability in the solvent could be proven. Mathematically the facts can be expressed with the Noyes-Whitney-Equation. [1,2,3]
Classic Comminution
The classical method used in earlier days used to be the manual comminution with a mortar. Today this process step is substantially alleviated with the automatic mortar mill. The adjustment of the most various parameters makes it possible to obtain reproducible results. This is almost impossible with a hand mortar since the application of energy fluctuates between users and can’t be constant. With the exact to the second adjustable grinding duration can the comminution with the Mortar Grinder PULVERISETTE 2 for example be easier integrated into the validation. Due to the gentle grinding only a slight rise in temperature is induced and therefore the sample in its basic structure not altered.
Innovative Comminution
But there are limits though in regards to the obtainable particle size. Due to the lower grinding energy only particles with a fineness of approximately 10 – 20 µm can be produced. The end of the dry grinding process is marked by the sticking of the samples. Here the Van der Waals forces and the electrostatic interplays of the small particles cause the agglomeration of the sample. In order to obtain a smaller particle size liquid is added in the next step in order to continue with the wet grinding. During this process, after a certain period a constant particle size will be obtained, since the particles in the suspension are not touched by the pestle any longer and float past it.
The innovative method of the Planetary Micro Mill PULVERISETTE 7 premium line allows a comminution far below the required range of particle size for chromatographic methods under consideration of important parameters like amount of liquid, grinding ball size as well as grinding duration and rotational speeds.
Fig 2.: Obtained particle size with the Mortar Mill PULVERISETTE 2 after 30 minutes of grinding (black curve) and achieved particle size of the Planetary Micro Mill PULVERISETTE 7 premium line after grinding 5 minutes (yellow curve).
The grinding technology in detail
 The sample is added along with the grinding balls in a grinding bowl in a planetary ball mill. The grinding bowls are fastened to a so called sun disk and turn counter clockwise around the centre of the disk. Due to impact, blow and friction effects of the grinding balls, the sample is comminuted effectively. The maximal possible rotational speed for planetary mills is limited to approximately 800 rpm. The decisive difference of the premium line to a conventional mill is the tensioning of the grinding bowls. Instead of fastening them to the sun disk, here the grinding bowls are lowered into the disk (SelfLOCK technology). This now allows a maximum rotational speed inside of the premium line of 1100 rpm and therefore an increase of the kinetic energy of the grinding medium by 150 %. The grinding duration into the nano range is drastically reduced respectively makes the comminution of nano particles for certain materials actually possible. [4]
The sample is added along with the grinding balls in a grinding bowl in a planetary ball mill. The grinding bowls are fastened to a so called sun disk and turn counter clockwise around the centre of the disk. Due to impact, blow and friction effects of the grinding balls, the sample is comminuted effectively. The maximal possible rotational speed for planetary mills is limited to approximately 800 rpm. The decisive difference of the premium line to a conventional mill is the tensioning of the grinding bowls. Instead of fastening them to the sun disk, here the grinding bowls are lowered into the disk (SelfLOCK technology). This now allows a maximum rotational speed inside of the premium line of 1100 rpm and therefore an increase of the kinetic energy of the grinding medium by 150 %. The grinding duration into the nano range is drastically reduced respectively makes the comminution of nano particles for certain materials actually possible. [4]
The most important parameters
 In order for the sample preparation to remain faultless, several rules have to be observed. The coarse comminution should always be conducted as a dry grinding. Only in the second step, with slight sticking of the produced powder, the liquid, the solvent is added. This is due to possible floating effects of the sample parts which would cause an inhomogeneous sample. Additionally, the amount of liquid for the wet grinding must be selected in regards to the sample material. The optimal comminution is achieved in liquid-pasty suspensions.
In order for the sample preparation to remain faultless, several rules have to be observed. The coarse comminution should always be conducted as a dry grinding. Only in the second step, with slight sticking of the produced powder, the liquid, the solvent is added. This is due to possible floating effects of the sample parts which would cause an inhomogeneous sample. Additionally, the amount of liquid for the wet grinding must be selected in regards to the sample material. The optimal comminution is achieved in liquid-pasty suspensions.
Processing the sample perfectly
Is the suspension too runny, the particles float past the grinding tools, in this case grinding balls. Is the suspension to viscous, the energy of the grinding tools are decelerated. The amount of liquid must always be corrected anew during the grinding process, since more and more new surfaces develop which must to be wetted. The critical temperature of the grinding sample should not be exceeded. Also the effect of the increased agglomeration with high temperatures must be observed.
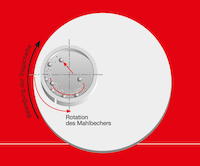
In further proceedings, in order to achieve a smaller final fineness, the grinding balls are exchanged step by step for smaller grinding balls. Here the degree of comminution should be checked via particle size analysis. The degree of comminution Z is a value from the chemical and mechanical process technology and offers information in regards how well a comminution process has worked.
It is defined as proportion of the largest particle diameter D in the base sample to the largest particle diameter d in the comminuted product. [1,2,3]
A comminution factor of 1 means that no comminution has occurred. If the largest particles, after comminution still measure half of their original diameter so is Z=2. The more forceful the comminution, the larger becomes n. So should the degree of comminution with constant temperature and exact solvent ratio in the further course barely change, so should the grinding balls be exchanged for the corresponding amount of smaller grinding balls. [3]
Sample analysis in practice
This comminution process can be utilized to examine antibiotics in regards to quality in accordance with the guidelines of U.S. Pharmacopoeia and European Pharmacopoeia. Examples of such supplements are gentamicin, neomycin, cefadroxil or bethanechol chloride. During the production inorganic contaminations (for example heavy metals) can occur. This detection of this possible contamination is an additional part of the analysis. With assistance of stripping voltammetry can the successfully comminuted supplement be examined for metal ions. These ions mostly originate from mercury containing thimerosal, which is utilized as a preserving agent for pharmaceuticals and cosmetics. The protection from microbial contamination is the reason for this. This compound is used for eye, nose and ear drops and tattoo ink, as well as cleaning and storage liquids for contact lenses. These delicate samples in their complex composition require a correct sample preparation in a system which conveys the samples in optimal conditions without falsifying them. [1]
Conclusion
The fast paced development of analytical methods in pharmaceutical technology requires always new adaptation of the comminution of the demanded parameters. In modern methods mostly the smallest obtainable particle size is of interest. Therefor the comminution mechanisms are continually optimized down into the nano range and matched to the characteristics of the corresponding samples.
In classical methods like the comminution with a hand mortar or mortar mill, the focus is more on the gentle comminution. Biological samples, like for example herbs or medicinal plants, would due the development of thermal effects caused by the high energy impact during nano grinding, lose their pharmacologically active substances. Therefore comminution technologies cannot be evaluated according to their efficiency, but instead a comminution technology must be selected according to task and analysis in order to obtain optimal results.
Is the emphasis only on the particle size though, so it has been shown that the latest advancements in the development of nano mills lead to much briefer grinding durations, where a particle size below 0.1 µm can be obtained.
Literature:
[1] K.H. Bauer, K.H. Frömming, C. Führer, Lehrbuch der Pharmazeutischen Technologie, 7. überarbeitete und erweiterte Auflage (2002)
[2] K. Hertwig, L. Martens, Chemische Verfahrenstechnik 2. überarbeitete Auflage, (2011)
[3] M. Stieß, Mechanische Verfahrenstechnik 2, 2., überarbeitete Auflage, (2011)
[4] www.fritsch.de
Author: techn. Laboratory Assistant Leos Benes, (TH Mittelhessen), E-Mail: benes@fritsch.de
Media Partners


