Channels
Special Offers & Promotions
Thermo Fisher Scientific Demonstrates Superiority of Data-Dependent Decision Tree Logic
 Thermo Fisher Scientific Inc., the world leader in serving science, today announced a new application note entitled "Enhancing Phosphotyrosine Proteome Coverage using a Combined ETD and CID Approach on a LTQ Orbitrap XL ETD". The application note is a result of the cooperation with the Netherlands Proteomic Centre and in particular the Biomolecular Mass Spectrometry and Proteomics Group at Utrecht University, headed by Albert Heck. It demonstrates how electron transfer dissociation (ETD) and collision induced dissociation (CID) are complementary fragmentation techniques, that when used together on the Thermo Scientific LTQ Orbitrap XL ETD mass spectrometer, provide significantly greater phosphoproteome coverage than mass spectrometry (MS) methods relying on CID alone. By using intelligent data-dependent decision tree (DDDT) logic to choose whether to perform ETD or CID in real-time, significantly more phosphopeptides can be identified, along with unambiguous determination of the phosphorylation sites. The application note is available for download via www.thermoscientific.com/orbitrap
Thermo Fisher Scientific Inc., the world leader in serving science, today announced a new application note entitled "Enhancing Phosphotyrosine Proteome Coverage using a Combined ETD and CID Approach on a LTQ Orbitrap XL ETD". The application note is a result of the cooperation with the Netherlands Proteomic Centre and in particular the Biomolecular Mass Spectrometry and Proteomics Group at Utrecht University, headed by Albert Heck. It demonstrates how electron transfer dissociation (ETD) and collision induced dissociation (CID) are complementary fragmentation techniques, that when used together on the Thermo Scientific LTQ Orbitrap XL ETD mass spectrometer, provide significantly greater phosphoproteome coverage than mass spectrometry (MS) methods relying on CID alone. By using intelligent data-dependent decision tree (DDDT) logic to choose whether to perform ETD or CID in real-time, significantly more phosphopeptides can be identified, along with unambiguous determination of the phosphorylation sites. The application note is available for download via www.thermoscientific.com/orbitrap
Reversible tyrosine phosphorylation plays an important role in many cellular processes such as growth, differentiation and migration. Because aberrant tyrosine phosphorylation has been shown to affect the course of many cancers, the detection and site-specific localization of tyrosine phosphorylation are important to understanding cancer mechanisms. Low abundances (compared with non-phosphorylated peptides) and the dynamic nature of tyrosine phosphorylation make it difficult to detect. A variety of strategies, including immuno-affinity enrichment of phosphoproteins and phosphopeptide enrichment prior to MS, have been developed to overcome underrepresentation of tyrosine phosphorylation. Despite advances in these approaches, characterization of the tyrosine phosphoproteome is far from comprehensive and ongoing method development is needed.
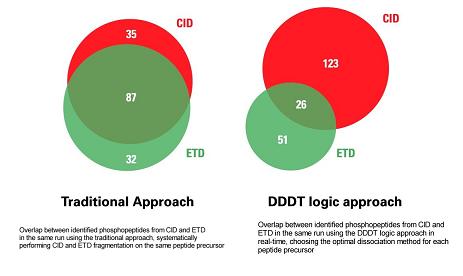
The application note compares two approaches to identify and localize tyrosine phosphorylation in a phospho-tyrosine peptide immuno-affinity purification of pervanadate treated HeLa cells. One is the traditional approach of fragmenting every peptide by both ETD and CID. The other approach is to use DDDT logic in real time to choose the most effective dissociation technique (ETD or CID) depending on the peptide's mass-to-charge ratio (m/z) and its charge state (z).
The LTQ Orbitrap XL ETDTM acquires high-resolution full-scan spectra in the OrbitrapTM mass analyzer, and then uses the linear ion trap to perform either ETD or CID. Its superior mass resolution enables DDDT logic to use the information in the full-scan spectra to make real-time decisions about which fragmentation method to use. Data was processed with the Thermo Scientific Proteome Discoverer software, which is specifically designed to handle raw data with CID and ETD spectra. It provides search algorithms for ETD data and combines results from multiple search engines and dissociation techniques.
Compared with the traditional approach, 30% more tyrosine phosphopeptides were identified when using intelligent DDDT logic. In both approaches, a significant number of phosphopeptides were identified only by ETD or CID, demonstrating that the techniques are truly complementary. ETD is most effective for peptides with three or more charges, while CID is most effective for those doubly charged. The nearly complete fragment ion series produced from complementary ETD and CID spectra generated using real-time DDDT logic enabled unambiguous determination of the tyrosine phosphorylation sites.
For more information or to download the application note, please call 1-800-532-4752 or visit www.thermoscientific.com/orbitrap
Thermo Scientific is part of Thermo Fisher Scientific, the world leader in serving science.
Media Partners


