Channels
Special Offers & Promotions
New data shows value of morphologically directed Raman spectroscopy for bioequivalence studies
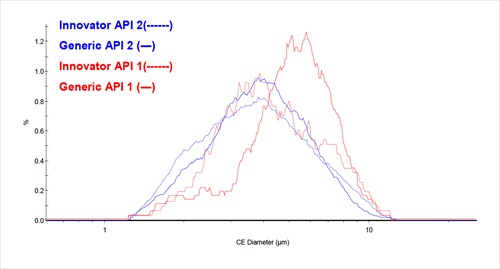
A new application note on the Malvern Instruments website describes the use of morphologically directed Raman microscopy in assessing the bioequivalence of a generic and innovator drug with a dual active pharmaceutical ingredient (API)
The work was carried out using the Morphologi G3-ID, which combines automated image analysis with Raman spectroscopy. The Morphologi G3-ID enables the determination of component-specific particle size distributions, which in this study were important because of the effects of particle size on tablet disintegration and subsequent bioavailability. ‘Generic versus Innovator: an In-Vitro Bioequivalence Study with the G3-ID’ is freely available as a download from the Malvern website.
In order to show that a generic drug is bioequivalent to an innovator it must display comparable bioavailability when studied under similar experimental conditions. Bioavailability is the rate and extent to which the active ingredient is absorbed from a drug product and becomes available at the site of drug action. Bioequivalence refers to equivalent release of the same drug substance from two or more drug products or formulations.
Morphologically directed Raman microscopy enables independent characterization of individual components present within a blend or mixture. As well as its application in bioequivalence studies, it can be used to gain better product understanding across many areas of the pharmaceutical industry from regulatory to troubleshooting. In delivering this capability, the fully automated Morphologi G3-ID is designed to allow both particle characterization scientists with limited spectroscopy experience and more experienced spectroscopists to get an in-depth understanding of their particulate samples.
Download ‘Generic versus Innovator: an In-Vitro Bioequivalence Study with the G3-ID’ at www.malvern.com/generic-vs-innovator
Malvern, Malvern Instruments, and Morphologi are registered trademarks of Malvern Instruments Ltd
Media Partners