Channels
Special Offers & Promotions
Introducing the new Alberta Idealized Throats from Copley Scientific - closer IVIVC in inhaled product testing
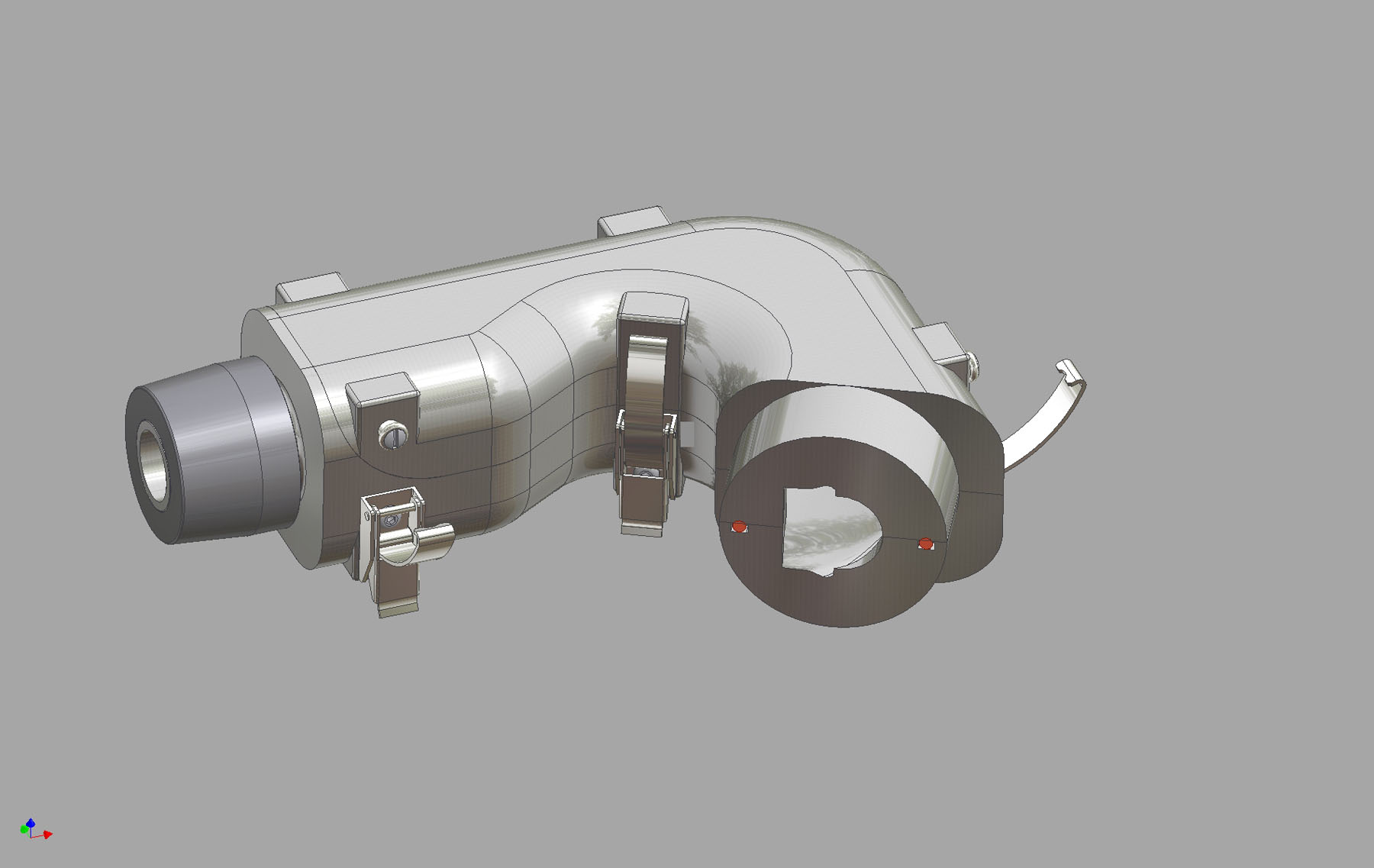 The Alberta Idealized Throat is a new product from Copley Scientific designed to improve in vivo/in vitro correlation (IVIVC) during inhaled product testing. Originally developed by the University of Alberta, Canada, and commercialized exclusively by Copley Scientific, the new throat is designed to provide a more ‘patient representative' alternative to the USP/Ph Eur induction port, routinely used for aerodynamic particle size measurement by cascade impaction. Experimental work confirms the ability of the new throat to more precisely reflect deposition behaviour in the human throat, thereby improving the relevance of test data within an R & D environment.
The Alberta Idealized Throat is a new product from Copley Scientific designed to improve in vivo/in vitro correlation (IVIVC) during inhaled product testing. Originally developed by the University of Alberta, Canada, and commercialized exclusively by Copley Scientific, the new throat is designed to provide a more ‘patient representative' alternative to the USP/Ph Eur induction port, routinely used for aerodynamic particle size measurement by cascade impaction. Experimental work confirms the ability of the new throat to more precisely reflect deposition behaviour in the human throat, thereby improving the relevance of test data within an R & D environment.
Achieving acceptable correlation between in vitro test methods and in vivo behaviour is a major challenge for the inhaled product sector. Research has shown that the USP/Ph.Eur. induction port that currently is widely used with cascade impactors for all inhaled products, can underestimate deposition in the human throat. Although highly effective as a simple QC tool, this compromises the value of the data for efficient R & D. The Alberta Idealized Throat was developed as a consequence of extensive research into typical patient populations, including the review of CT scans and anatomy texts.
The Alberta Idealized Throat has standardised, highly reproducible, human-like geometry, offering robust performance that is independent of flow rate. The Alberta Highly Idealized Throat has a smooth, more uniform internal geometry than a human throat cast, making drug recovery much simpler. This is further aided by a two-piece construction, utilising quick- release clips for easy internal access.
For more details visit www.copleyscientific.com
Media Partners


