Channels
Special Offers & Promotions
Clinical Trial Launches to Develop Breath Test for Multiple Cancers
Study will use Owlstone Medical’s Breath Biopsy platform to identify breath biomarkers to detect and differentiate early-stage disease
 Owlstone Medical a global diagnostics company developing a breathalyzer for applications in early disease detection and precision medicine, and Cancer Research UK, announces the clinical launch of the PAN Cancer trial for Early Detection of Cancer in Breath1, with the first patients now being recruited for the 1,500 patient study.
Owlstone Medical a global diagnostics company developing a breathalyzer for applications in early disease detection and precision medicine, and Cancer Research UK, announces the clinical launch of the PAN Cancer trial for Early Detection of Cancer in Breath1, with the first patients now being recruited for the 1,500 patient study.
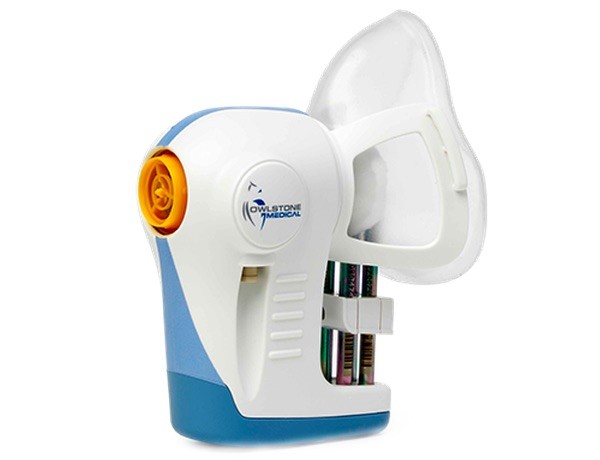
As previously announced on initiation of the collaboration, researchers in the trial are using Owlstone Medical’s2 Breath Biopsy® platform to collect samples, including healthy individuals as trial controls, to analyze volatile organic compounds (VOCs) in the breath. The trial aims to identify VOCs that can be used as breath-based biomarkers to detect and differentiate different cancer types, thereby improving early detection.
This approach holds great promise as cancer cells display altered metabolism even at the very earliest stage of disease, affecting the pattern of the VOCs exhaled. By identifying the changing pattern of VOCs early, Owlstone Medical’s Breath Biopsy technology could detect cancer at the earliest stage of disease, when treatments are more effective and more lives can be saved.
The trial is recruiting 1,500 patients at Addenbrooke’s Hospital in Cambridge who have been referred from their GP with these specific types of suspected cancer. Prior to other diagnostic tests, breath samples will be collected using Owlstone Medical’s ReCIVA® Breath Sampler and processed in Owlstone Medical’s Breath Biopsy laboratory in Cambridge, UK.
The clinical trial has initiated in patients with suspected oesophageal and stomach cancers and will expand to prostate, kidney, bladder, liver and pancreatic cancers in the coming months. Research is anticipated to run through 2021, and if the technology proves to accurately identify cancer the team hope that breath biopsy could in the future be used routinely in GP practices to determine whether to refer patients for further diagnostic tests.
Professor Rebecca Fitzgerald, lead trial investigator at the Cancer Research UK Cambridge Centre, said: “We urgently need to develop new tools, like this breath test, which could help to detect and diagnose cancer earlier, giving patients the best chance of surviving their disease. Through this clinical trial we hope to find signatures in breath needed to detect cancers earlier – it’s the crucial next step in developing this technology. Owlstone Medical’s Breath Biopsy technology is the first to test across multiple cancer types, potentially paving the way for a universal breath test.”
Billy Boyle, co-founder and CEO at Owlstone Medical, said: “There is increasing potential for breath-based tests to aid diagnosis, sitting alongside blood and urine tests in an effort to help doctors detect and treat disease. The concept of providing a whole-body snapshot in a completely non-invasive way is very powerful and could reduce harm by sparing patients from more invasive tests that they don’t need.
“Our technology has proven to be extremely effective at detecting VOCs in the breath, and we are proud to be working with Cancer Research UK as we look to apply it towards the incredibly important area of detecting early-stage disease in a range of cancers in patients.”
Almost half of cancers are diagnosed at a late stage in England3. This highlights the importance of early detection, particularly for diseases like oesophageal cancer where only 12% of oesophageal cancer patients survive their disease for 10 years or more. For example, Rebecca Coldrick, 54 from Cambridge, was diagnosed in her early 30s with Barrett’s oesophagus, a condition where the cells lining the oesophagus are abnormal – often caused by acid reflux. Out of 100 people with Barrett’s oesophagus in the UK, up to 13 could go on to develop oesophageal adenocarcinoma4.
Rebecca Coldrick said: “About 20 years ago I developed acid reflux, and I began to live on Gaviscon and other indigestion remedies. I went to the doctors and shortly after I was diagnosed with Barrett’s. Every two years I have an endoscopy to monitor my condition.”
Dr David Crosby, head of early detection research at Cancer Research UK, said: “Technologies such as this breath test have the potential to revolutionize the way we detect and diagnose cancer in the future. Early detection research has faced an historic lack of funding and industry interest, and this work is a shining example of Cancer Research UK’s commitment to reverse that trend and drive vital progress in shifting cancer diagnosis towards earlier stages.”
Recognising the importance of early detection in improving cancer survival, Cancer Research UK has made research into this area one of its top priorities and will invest more than £20 million a year in early detection research by 2019.
-
While Owlstone will be funding the trial directly, none of this would be possible without the support and infrastructure provided by Cancer Research UK. The PAN Cancer trial is being conducted in collaboration with a team of leading cancer researchers at the Cancer Research UK Cambridge Centre, the University of Cambridge and Cambridge University Hospitals NHS Foundation Trust. The Chief Investigator is Professor Rebecca Fitzgerald, who is co-lead of the Cancer Research UK Cambridge Centre Early Detection Programme, Professor of Cancer Prevention at the MRC Cancer Unit, and an Honorary Consultant in Gastroenterology and General Medicine at Addenbrooke's Hospital, Cambridge.
-
For all cancers (excluding non-melanoma skin cancer) diagnosed at a late stage (3 or 4) of those with a known stage at diagnosis in England (2016). Source: http://www.ncin.org.uk/publications/survival_by_stage
-
Gatenby P, Caygill C, Wall C, et al. Lifetime risk of esophageal adenocarcinoma in patients with Barrett's esophagus. World J Gastroenterol 2014;20(28):9611-7.
Media Partners