Channels
Special Offers & Promotions
CloudLIMS Adds a HIPAA-Compliant Patient Portal to its COVID-19 LIMS for Diagnostic Labs to Offer a Seamless Experience to Testees
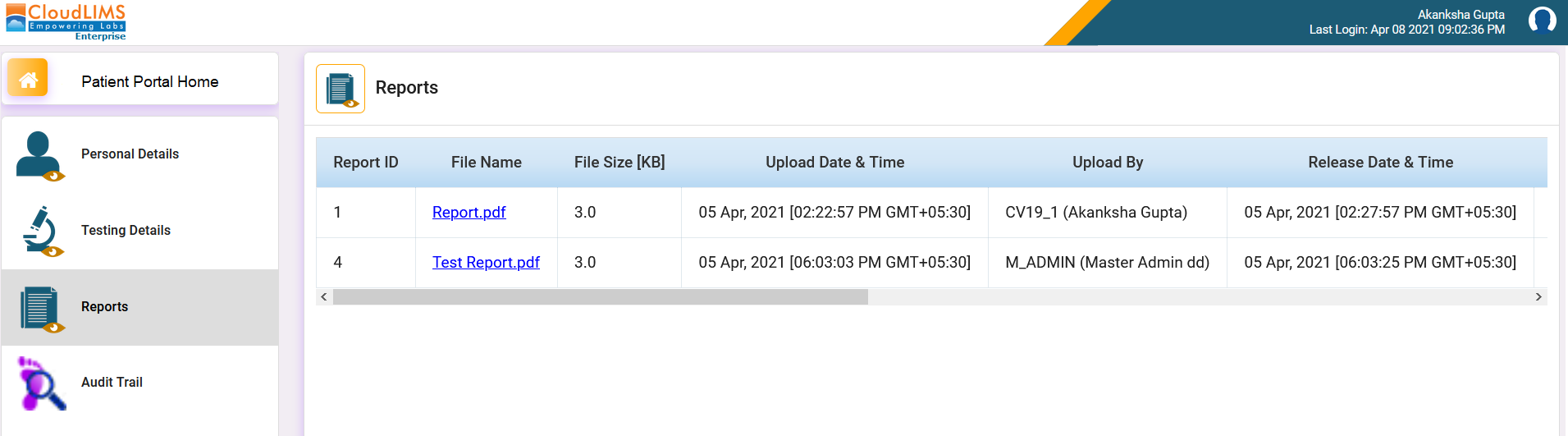
CloudLIMS’s cloud-based, HIPAA-compliant patient portal enables clinical and COVID-19 diagnostic laboratories to register & securely deliver test reports to testees.
CloudLIMS, a leading provider of laboratory informatics, announces the release of a HIPAA-compliant, cloud-based, secure patient portal to enable clinical and COVID-19 diagnostic laboratories to offer direct-to-patient services for seamless communication. The patient portal is included in the latest release of CloudLIMS, a full-feature, out-of-the-box, COVID-19 LIMS.
This functionality enables testees to self-register on a secure cloud-based portal prior to sample collection, allows efficient check-ins, tracks the status of their test requests, and enables them to download test reports as soon as they are released by the diagnostic laboratory. Furthermore, the patient portal enhances the participation of testees in their healthcare, allows them to download, print, and share their reports, and enables laboratories to offer exceptional customer service.
"We developed a patient portal to enable clinical and COVID-19 diagnostic laboratories to offer a seamless experience to the testee. The portal enables testees to pre-register to avoid delays in sample collection and waiting time, provides tracking of testing status, and offers instant access to their test reports securely," said Arun Apte, Chief Executive Officer at CloudLIMS. "We aim to bridge the communication between testees and diagnostic laboratories, enhance the experience of testees and enable laboratories to offer direct-to-patient services," he continued.
Video - COVID-19 LIMS Software for Coronavirus Diagnostic Testing Labs
About CloudLIMS
CloudLIMS.com is an ISO 9001:2015 certified informatics company. Their SaaS, in the cloud Laboratory Information Management System (LIMS), CloudLIMS, offers strong data security, complimentary technical support, instrument integration, hosting and data backups to help biorepositories, analytical, diagnostic testing and research laboratories, manage data, automate workflows, and follow regulatory compliance such as CLIA, ISO 15189:2012, HIPAA, ISO/IEC 17025:2017, GxP, 21 CFR Part 11, ISO 20387:2018, and ISBER Best Practices at zero upfront cost.
Media Partners


