Channels
Special Offers & Promotions
Targeted Hyperthermia Treatment Accepted for Full Characterization at the Frederick National Laboratory for Cancer Research
Siva Therapeutics (“Siva”) is pleased to announce that its Targeted Hyperthermia™ technology has successfully passed through the first stages of nanoparticle characterization at the Frederick National Laboratory (FNL) for Cancer Research in Frederick, Md.
FNL’s Nanotechnology Characterization Laboratory (NCL) – a partnership with the U.S. Food and Drug Administration and the National Institute of Standards and Technology – is a world leader in the characterization of therapeutic nanoparticles, which helps pave the way for regulatory review of nanotechnologies intended for cancer therapies and diagnostics.
The NCL uses a three-tiered assay cascade to thoroughly characterize each nanomaterial strategy submitted to the lab for physical and chemical attributes, in vitro biological properties, and in vivo compatibility through preclinical toxicology, pharmacology, and efficacy studies. Through an application process, NCL offers preclinical characterization services to investigators nationwide who have developed promising nanotechnology-based cancer treatments. NCL’s assay cascade takes about a year to complete.
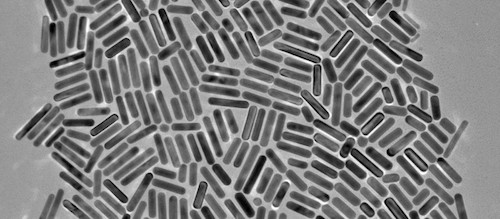
Siva delivered a pilot-scale batch of SivaRods™ precision gold nanorods, manufactured by partner company NanoHybrids, Inc. to the NCL early in 2017. “We are pleased that our partnership with Siva continues to progress and build value for both companies,” stated Kimberly Homan, PhD,CTO of NanoHybrids. “Through the NCL process, we are building support for the clinical translation of Siva’s promising technology.”
“The NCL Assay Cascade Program is the premier program in the world for bringing nanomaterials through critical preclinical stages and facilitating regulatory review.” said Len Pagliaro, PhD, CEO of Siva. “We are very pleased at having successfully passed through the first stages of the program, a step which validates the manufacturing capabilities of our partners at NanoHybrids, Inc., and the commercial potential of SivaRods for Targeted Hyperthermia. The scientific, technical, and regulatory resources of the NCL are greatly strengthening Siva’s next steps toward the clinic.”
About NanoHybrids Inc.
NanoHybrids develops nanotechnology solutions that facilitate non-invasive detection, molecular profiling, and novel treatments for disease. NanoHybrids combines a clinical development pipeline with commercially available products and services to drive theranostic nanoparticle innovations across applications spanning biomedical imaging and drug delivery. Through academic and industrial partnerships, the company strives to enhance the convergence of nanotechnology and medicine. Contact us today info@nanohybrids.net or visit us online at www.nanohybrids.net
About Siva Therapeutics Inc
Siva Therapeutics Inc is developing Targeted Hyperthermia™, a photothermal cancer therapy, which uses therapeutics heat to treat solid cancers. The heat is delivered to tumors by infrared light that is absorbed by SivaRods™ gold nanorods and re-emitted as heat. The size, shape, and surface chemistry of the nanorods target the leaky vasculature of solid tumors, and the selective thermal sensitivity of tumor tissue enables the therapy to deliver clean margins. Targeted Hyperthermia promises to be extremely safe, effective, competitive in cost, and a valuable adjunct to drug therapy and other cancer treatments.
Contact us at info@sivatherapeutics.com or visit: www.sivatherapeutics.com
About the Nanotechnology Characterization Laboratory
The Nanotechnology Characterization Laboratory (NCL) accelerates the development of nanotechnology for basic and applied cancer research. It is a national resource for cancer researchers to test nanotechnologies intended for cancer therapies and diagnostics. The NCL aims to reduce suffering and death from cancer by accelerating the transition of basic nanoscale particles and devices into clinical applications. NCL is part of the Frederick National Laboratory for Cancer Research, sponsored by the National Cancer Institute.
Media Partners