Channels
Special Offers & Promotions
Waters Simplifies the Clinical Data Review Process with New waters_connect™ for IVD and QUAN Review Application
Waters announces the release of waters_connect™ for In Vitro Diagnostic (IVD), a software platform designed for maximum data integrity, compliance, security, and accessibility that provides a backbone for clinicians to securely share data.
With QUAN Review, a waters_connect™ for IVD application, laboratories can now simplify their clinical LC-MS data processing with a layered approach to data analysis in an easy-to-use software package.
Introduced at the Mass Spectrometry and Advances in the Clinical Lab (MSACL) 2024 14th Annual Conference & Exhibits in March, waters_connect™ for IVD software and QUAN Review application are available now in the United States, the United Kingdom, Canada, and Europe.
Key features of the Waters QUAN Review Application include:
- Reduce data review time by up to 50% (as compared to Waters TargetLynx™ Software).
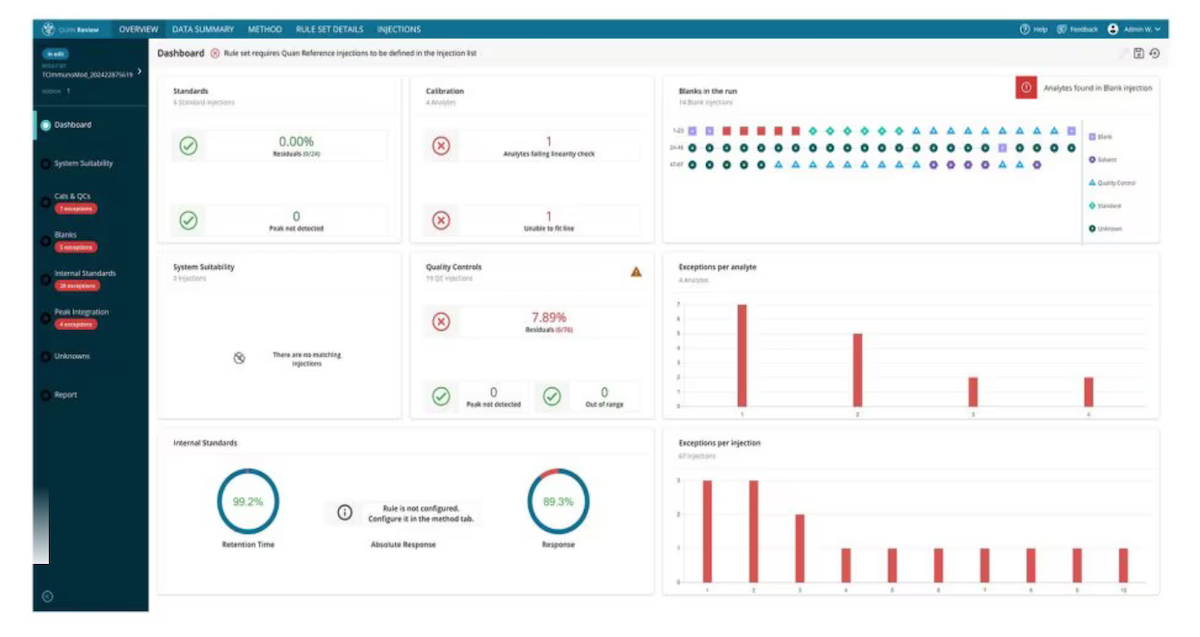
- Offers a one-screen dashboard that summarizes key parameters such as standards, calibration, and quality control (QC) – grouped together for a streamlined view.
- Allows users to easily see and quickly address errors that need attention via Exception-Focused Review (xfR).
- PanoGramic Display, a view that enables users to quickly identify problems or ambiguous data by showing multiple chromatograms, calibrations, and QC at the same time.
- Built-in connectivity to the user’s laboratory information management system (LIMS).
The software allows laboratories to set flags or alerts according to their standard operating procedures (SOPs). Additionally, users can:
- Leverage the default result set to highlight data that is out of range such as R2, standard deviation or signal-to-noise values.
- Select which rules are to be applied and the range/tolerance for each (e.g., the internal standard must be within 20% of the median response).
- Reduce turnaround time for labs with high sample volume and complex data sets.
For staff in the clinical laboratory, faster, more efficient data review means fewer errors, quicker turnaround times, and reduced manual labor, which enables staff to focus on value-added tasks.
About Waters Corporation
Waters Corporation (NYSE:WAT), a global leader in analytical instruments and software, has pioneered chromatography, mass spectrometry, and thermal analysis innovations serving the life, materials, and food, and environmental sciences for more than 65 years. With approximately 7,900 employees worldwide, Waters operates directly in more than 35 countries, including 15 manufacturing facilities, and with products available in more than 100 countries.
Waters, waters_connect, and TargetLynx™ are trademarks of Waters Technologies Corporation.
Recent news from Waters Corporation
Media Partners


