Members Login

Channels
Special Offers & Promotions
Bioplastics Research Aided by CDS Analytical Pyrolyzers
One of the newest, and most promising, developments in polymer science is the emergence of bioplastics. Bioplastics are polymers made completely, or at least partially, from renewable resources like cellulose, starch, and algae. It is widely believed that increasing acceptance of bioplastics will help slow the pace of climate degradation by reducing dependence on fossil fuels. The market for bioplastics is accelerating rapidly and industry experts believe it could reach $3 billion by 2018.
Pyrolysis-GCMS has long played a role in the study of industrial polymers such as rubbers, coatings, and plastics. The technique is admired for both qualitative and quantitative effectiveness when used to identify and understand unknown polymers and additives. This familiar, tested technology is now being used to assist in the identification of unknown bioplastics, as well as research targeting new materials. Analytical pyrolysis of these newer plastics can reveal their true makeup.
A common biopolymer is polylactic acid (PLA), a plastic made from cornstarch. When this plastic is pyrolyzed, you will get a prominent peak for PLA's dimer (lactide). Another example is Polyhydroxybutyrate (PHB), which is made by bacteria. When this biopolymer is pyrolyzed, the main peak produced that we can use for identification is butenoic acid.
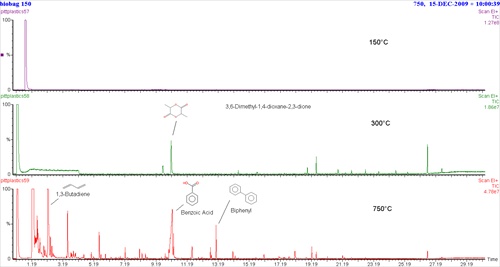
As would be expected, depending on sample complexity, not all biopolymers are as easy to identify. However, with some of the recent advances in pyrolysis instruments and polymer identification libraries, it is now possible to identify trace level additives and polymers in the most complex multi-polymeric samples. Some newer pyrolyzer systems have the ability to heat a given sample at multiple temperatures, making it possible to "thermally cut" analytical results into simpler, less complex runs.
The example shown here is from a compostable plastic garbage bag run at three different temperatures, 150°C, 300°C and 750°C. Results from the second run (300°C) show that a small amount of lactide is produced indicating PLA, which is usually made from cornstarch. Having the option of multiple-step runs made it possible to locate the lactide, hidden under the large peak for benzoic acid in the 3rd highest temperature run. It can be detected here by extracting ion 56, but without seeing it in the lower temperature (300°C) run first, it might easily have been missed. The last pyrolysis run (750°C) also produces a large peak for benzoic acid, and another peak for biphenyl. These are aromatics typical of polyesters containing terephthalate. Butadiene is also present, so the combination of these pyrolysis products could result from the compostable polyester, polybutylene adipate/terephthalate, in addition to PLA seen in the second run (300°C).
CDS Analytical manufactures a full product line of pyrolyzers and GCMS injection systems. We will be displaying our products at the upcoming PITTCON, Analytica-Munich and Arab Lab conferences. You can also review our pyrolyzer line on our web page at http://www.cdsanalytical.com/instruments/pyrolysis/pyroprobe_5000.html .
For further application details on pyrolysis of bioplastics or other polymer applications, please contact CDS at sales@cdsanalytical.com or call at +1 610 932 3636.
Media Partners


