Channels
Special Offers & Promotions
Two new weapons in the battle against bacteria - alternative approaches to antibiotics development
Two new weapons in the battle against bacteria
Proteases are vital proteins that serve for order within cells. They break apart other proteins, ensuring that these are properly synthesized and decomposed. Proteases are also responsible for the pathogenic effects of many kinds of bacteria. Now chemists at the Technische Universitaet Muenchen (TUM) have discovered two hitherto unknown mechanisms of action that can be used to permanently disarm an important bacterial protease.
Proteins are made up of a chain of amino acids and are vital for all cell processes. Proteases are among the most important types of protein. Like “molecular scissors”, they cut other proteins at given positions and thereby execute important cell functions. By cutting the amino acid chains to the right length or breaking proteins apart they, for example, activate or deactivate proteins, decompose defective ones or switch signal sequences that serve to transport proteins to their proper position within a cell.
But proteases are important not only for human cells – bacteria also rely on them. There are hardly any effective antibiotics left in the fight against pathogens like multi-resistant strains of Staphylococcus aureus bacteria or Mycobacterium tuberculosis which causes tuberculosis.
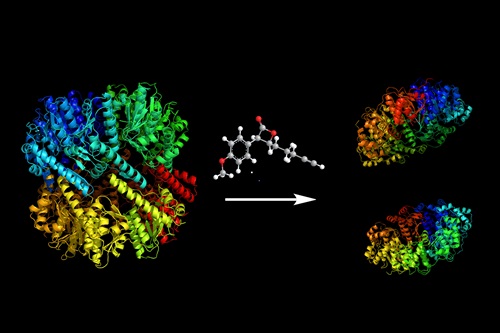
Researchers around the world are thus working ardently to find new ways of disarming the proteases in these strains to combat them. At the heart of this effort lies the so-called ClpP protease. It comprises 14 subunits and has a central regulatory function. The usual approach to deactivating this protease is to block all active centers of the ClpP. These are effectively the “cutting edges of the scissors”, i.e. the portions of the protein that are responsible for breaking apart other proteins.
“However, the inhibitors used in the past have one decisive disadvantage,” explains Stephan Sieber who heads the Chair for Organic Chemistry II at the Technische Universitaet Muenchen (TUM). “They don’t permanently disarm the proteins, but only work for a few hours. On top of that, to be effective they must attack all active centers of the protein.”
New strategies against bacteria
In collaboration with Professor Michael Groll, who heads the Chair for Biochemistry, Malte Gersch and Roman Kolb, doctoral candidates at Professor Sieber’s chair, have succeeded in uncovering two completely new mechanisms that can be used to permanently deactivate these important bacteriological proteases – in one case even without having to attack all active centers of the protein.
The first mechanism disrupts the arrangement of amino acids required for the cohesion of the protease subunits. As a result the protease breaks into two parts. The second acts directly on the core of the active center. It converts the amino acid that does the actual splitting into another kind of amino acid – the “scissors” lose their edge and the protein is rendered inoperable. Both approaches inhibit the protease in completely novel ways and are thus very promising for the development of new forms of medication.
The scientists also found a whole series of inhibitors that initiate the two mechanisms. “Knowing the ways in which substances deactivate the proteases is a huge advance,” says Gersch. “We can now optimize the substances and possibly also apply the principle to other proteases.”
In further research, Gersch and Sieber plan to test their substances on living bacterial strains to determine if these are truly inhibited in growth and pathogenic effect. “Although the bacteria are not completely disarmed, they produce significantly fewer toxins that are conducive to inflammation,” says Gersch. “The basic idea is that we give the immune system more time to handle the pathogens on its own while the formation of new resistances is suppressed.”
The research was funded by the Deutsche Forschungsgemeinschaft (DFG; SFB 749 and SFB 1035, Cluster of Excellence Center for Integrated Protein Research Munich (CIPSM)), by the European Research Council (ERC starting grant), The Fund of the Chemical Industry and the German National Academic Foundation. The crystal structures were determined in collaboration with the synchrotron radiation source of the Paul Scherrer Institute in Villigen (Switzerland).
more about Technische Universitaet Muenchen
Media Partners