Channels
Special Offers & Promotions
Matrix Gemini EM Facilitates Environmental Monitoring in Pharmaceutical, Biotechnology or Medical Device Manufacture
Environmental Monitoring is an essential part of any pharmaceutical, medical device or biotechnology manufacturing process in order to show that the microbial and particulate content of all clean room air and work surfaces is below acceptable levels.
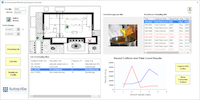 Matrix Gemini EM from Autoscribe Informatics provides all the functionality to define a complete environmental monitoring protocol. It can be used in an FDA regulated environment and fully supports the requirements of 21CFR Part 11.
Matrix Gemini EM from Autoscribe Informatics provides all the functionality to define a complete environmental monitoring protocol. It can be used in an FDA regulated environment and fully supports the requirements of 21CFR Part 11.
Matrix Gemini EM is a specific configuration of the very successful Matrix Gemini LIMS (Laboratory Information Management System). It will support a typical EM workflow “out of the box”, but can easily be configured to match the exact needs of different laboratories. The entire process from sample planning and testing schedule to reporting is done in a secure, compliant system.
In order to support an EM workflow, Matrix Gemini EM can associate a group of sampling sites within multiple Controlled Environments (CE), together with the tests and test schedule for each site. It also provides the screens necessary to collect all sample results and meta-data important to microbiological tests. Once a sampling event is scheduled, labels are printed to speed sample collection and ensure that each sample point is collected.
One or more tests are then assigned to each sample. Tests contain a result entry grid that is defined by the user along with limits (specifications). Work is then assigned to individuals, incubation dates are entered, and the samples are marked as collected. If results are produced on an automated reader, they may be transferred to the Matrix database electronically, thus speeding the process and eliminating paperwork and transcription errors. Any violation of alert limits or action limits is notified immediately.
Once the results are validated then reports can be generated very quickly and easily, including trend plots filtered by selectable fields, without passing the information to a separate, non-validated spreadsheet program. Possible trend plots include: for a specific room (CE) over a chosen time, for a specific batch/lot, or by microbiologist to identify training needs, and many more.
Media Partners


