Channels
Special Offers & Promotions
Australia
Retinal implant innovator Bionic Vision Technologies (BVT) has announced it had raised US $18 million (AU$23.5 million) from Hong Kong-based China Huarong International Holdings Ltd and State Path Capital Limited, to develop and commercialise its next generation devices aimed at restoring vision to the blind.
 This financing formally launches the Company as it transitions to a commercialisation stage business. To date, the development of Australia’s ‘bionic eye’ has been funded through a five year AU$50 million Special Research Initiative grant administered by the Australian Research Council.
This financing formally launches the Company as it transitions to a commercialisation stage business. To date, the development of Australia’s ‘bionic eye’ has been funded through a five year AU$50 million Special Research Initiative grant administered by the Australian Research Council.
BVA consortium members, whose organisations become alongside the new investors, comprise of the University of Melbourne, the University of New South Wales, the Bionics Institute, Centre for Eye Research Australia, CSIRO’s Data 61, The Royal Victorian Eye & Ear Hospital, Western Sydney University and the Australian College of Optometry.
BVT will use the new funds to manufacture devices and begin a human clinical trial of its ‘bionic eye’ implant in patients with the inherited degenerative eye condition called retinitis pigmentosa. The condition is the most common cause of inherited blindness and affects more than 1.5 million people worldwide.
The investment was made on the basis of BVT’s advanced work in developing bionic vision technologies including a successful clinical trial of a prototype device in three patients. BVT’s bionic eye has a number of significant advantages over competitors, including a superior surgical approach, stability of the device, and unique vision processing software that potentially improves the patient’s experience.
State Path Capital Limited Chairman Mr Alastair Lam said: “Given BVT's commitment to developing and delivering a revolutionary solution for vision loss, we believe its ‘bionic eye’ technology has the potential to transform the lives of millions of people and meet a large unmet need. Our investment support will help move the current product closer to market and the communities who will benefit."
“Our investment in BVT aligns with our strategy of backing transformative new technology with significant global potential,” said Mr Lam, nephew of Hong Kong-based business magnate, investor and philanthropist Sir Li Ka-shing.
Bionic Vision Technologies Executive Chairman Robert Klupacs said: “This investment is an important milestone for our unique Australian technology and an endorsement of our approach to making a positive impact on global health. These new funds will help create an innovative, solution to potentially help improve the lives of blind people.”
“The funding will propel this Australian technology into clinical trials in coming months as we work towards securing regulatory approval and a commercial launch in key markets where loss of vision is a significant medical burden.”
“There is currently no treatment for conditions such as retinitis pigmentosa and our new investors recognise BVT has developed a world-leading solution with potential to make a significant impact patient’s sight and lifestyle.”
The National Eye Institute, US, estimates that 1 in 4,000 people have retinitis pigmentosa worldwide with a higher number of cases in China and India compared to other countries in the developed world.
The development of a retinal implant will draw on BVT’s expertise and track record of success in this field.
The bionic eye consortium was a finalist in the 2013 Eureka Awards and the 2013 Melbourne Awards. Many of its researchers have won prestigious prizes for their outstanding research including some named in the top 100 most influential engineers in Australia, and by the Knowledge Society and the Office of the Chief Scientist in the Knowledge Nation 100 top innovators in science and technology.
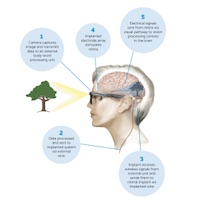
The unique implant BVT has created is placed at the back of the eye where it stimulates the surviving nerve cells in the retina with electrical signals created from images collected by an externally worn camera. Next stage clinical trials are scheduled to start in coming months in Melbourne.
Based on the results of the initial trial in 2012-2014, further patients will be recruited and monitored for up to two years. Patients will be surgically implanted with a permanent device to wear in their everyday activities. Early trials of the device only monitored patients in the clinic. Researchers will measure mobility and independence.
more about bionic vision technologies
Media Partners


