Channels
Special Offers & Promotions
ATCC Adds New Customer-driven Format to its Quality Control Standards Portfolio
Makes it easier for industrial and pharmaceutical labs to test for product safety
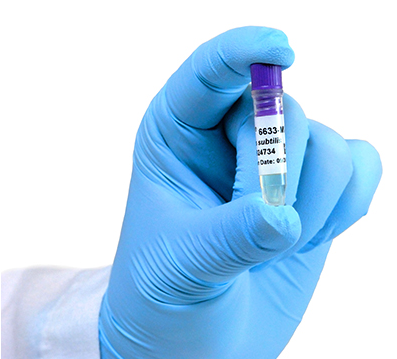 ATCC, the premier global biological materials resource and standards organization, announces the release of ATCC® Minis to support quality control (QC) testing in pharmaceutical and industrial labs, during the PDA 9th Annual Global Conference on Pharmaceutical Microbiology in Bethesda, MD,
ATCC, the premier global biological materials resource and standards organization, announces the release of ATCC® Minis to support quality control (QC) testing in pharmaceutical and industrial labs, during the PDA 9th Annual Global Conference on Pharmaceutical Microbiology in Bethesda, MD,
Healthcare, personal care product, and cosmetic manufacturers are required to test the bio-burden and sterility of their products and production environments to ensure consumer safety. Global alignment and harmonization of microbial testing requirements among the United States Pharmacopeia (USP), Japanese Pharmacopeia (JP), and European Pharmacopeia (EP), have resulted in the need for consistent and reliable control organisms at less than five passages from the ATCC reference stock for reproducible results.
“Many customers spend considerable time and resources creating cryopreserved stocks from ATCC organisms, acquired either directly from ATCC and our exclusive distributors or through ATCC Licensed Derivative® program partners. With the availability of ATCC® Minis, we now provide our high quality strains in a ready to use mini-cryovial format to meet customers’ requirements,” said Dr. Mindy Goldsborough, ATCC Vice President. “We have created single-use glycerol stocks, so our customers can validate their QC work quickly and efficiently without the risk of cross-contamination during banking techniques.”
Only ATCC Genuine Cultures® are authenticated and supported by ATCC polyphasic testing to ensure both microbial identity and phenotypic characteristics. To meet the needs of QC biologists, ATCC developed ATCC® Minis – with the same high-quality ATCC Genuine Cultures® – provided as a six-pack of ready-to-use QC strains preserved in glass-free “mini” cryovials containing glycerol stock. Each tube has a 2D barcode to allow for easy storage and tracking, and offers a peel-off label for fast and reliable recordkeeping.
Today, ATCC has released the following ATCC® Minis for the top 12 strains used in QC testing, including those that support test methods for non-sterile products, such as USP 61 and USP 62: Pseudomonas aeruginosa (ATCC® 9027-MINI-PACKTM), Staphylococcus aureus (ATCC® 6538-MINI-PACKTM), Candida albicans (ATCC® 10231-MINI-PACKTM), Aspergillus brasiliensis (ATCC® 16404-MINI-PACKTM), Escherichia coli (ATCC® 8739-MINI-PACKTM), Bacillus subtilis (ATCC® 6633-MINI-PACKTM), Salmonella enterica (ATCC® 14028-MINI-PACKTM), Escherichia coli (ATCC® 25922-MINI-PACKTM), Staphylococcus aureus (ATCC® 25923-MINI-PACKTM), Pseudomonas aeruginosa (ATCC® 27853-MIN-PACKTM), Enterobacter aerogenes (ATCC® 13048-MINI-PACKTM), and Enterococcus faecalis (ATCC® 29212-MINI-PACKTM). In addition, ATCC is offering convenient ATCC® Minis accessories and a QC pack of the USP recommended ATCC organisms.
About ATCC
ATCC is an innovative global partner to the scientific community for authenticated biomaterials, quality standards, and custom services. With the world’s largest and most diverse collection of human, animal, and plant cell lines, as well as molecular genomic tools, microorganisms, and biological products, ATCC is the most respected and trusted resource for the worldwide research community. ATCC associates share in its mission to acquire, authenticate, preserve, develop, and distribute biological materials and information to advance better science.
Media Partners


