Channels
Special Offers & Promotions
Freeze-Drying Microscope Generates Critical Formulation Specific Data
SP Scientific has video interviewed Dr Kevin Ward of Biopharma Process Systems Ltd, to discuss the key benefits of the Lyostat5 Freeze-Drying Microscope (FDM), and how it can be used to generate critical formulation-specific data
Every freeze-drying formulation has a critical temperature, below which it should be maintained during primary drying in order to prevent processing defects.
Dr Ward explains how using the Lyostat5 freeze drying microscope allows observation of the sample structure during drying and precise temperature control when cooling or heating so that the exact point of collapse can be determined. In addition, he describes how the Lyostat5 freeze-drying microscope lighting and image capture systems provide sharp visualisation for easy, accurate identification of structural changes. Using the derived data, he explains how after analysis has been carried out that formulations and cycles can be developed that are safe, robust, and cost efficient. Dr Ward summarises that for every 1°C higher a formulation can be freeze dried at, then results in an efficiency saving of 13%.
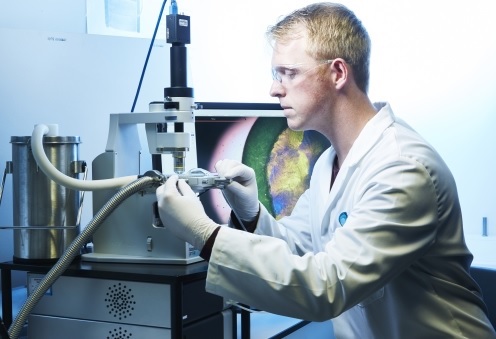 The Lyostat5 freeze drying microscope is part of the SP Scientific Line of Sight™- enabled suite of freeze drying equipment and technologies portfolio. As well as enabling the determination of critical temperatures including eutectic melting temperature (Teu) and collapse temperature (Tc), the Lyostat5 freeze drying microscope also enables the identification of crystallization phenomena, skin/crust formation, and the effects of annealing on ice crystal growth and solute structure. Knowledge of the critical temperatures is essential to conduct cycle development on a rational, quantitative scientific basis as well as saving precious resources in terms of time and monies needed. The Lyostat5 freeze-drying microscope is supplied fully validated as a complete working system against a series of standard solutions.
The Lyostat5 freeze drying microscope is part of the SP Scientific Line of Sight™- enabled suite of freeze drying equipment and technologies portfolio. As well as enabling the determination of critical temperatures including eutectic melting temperature (Teu) and collapse temperature (Tc), the Lyostat5 freeze drying microscope also enables the identification of crystallization phenomena, skin/crust formation, and the effects of annealing on ice crystal growth and solute structure. Knowledge of the critical temperatures is essential to conduct cycle development on a rational, quantitative scientific basis as well as saving precious resources in terms of time and monies needed. The Lyostat5 freeze-drying microscope is supplied fully validated as a complete working system against a series of standard solutions.
For further information on the Lyostat5 freeze drying microscope, please contact SP Scientific on +1-845-255-5000 (+44-1473-240000 in Europe).
About SP Scientific
SP Scientific is a leading manufacturer of freeze dryers/lyophilizers, aseptic vial washing and tray loading machines, temperature control/thermal management, centrifugal evaporators and concentrators, glassware washers, and controlled environmental rooms and chambers. The company sells its products under well-known brands including VirTis, Hull, PennTech, FTS Systems, Genevac and Hotpack. SP Scientific has ISO 9001:2015 registered production facilities in the USA and Europe.
Line of Sight™ is a breakthrough concept from SP Scientific comprising a suite of freeze-drying equipment with scalable lyophilization technologies and process analytical tools (PAT). Line of Sight has been designed for pharmaceutical developers and manufacturers to assist them achieve drug commercialization objectives. Line of Sight technologies have been developed to provide the tools for a more successful scale-up of a lyophilization process from formulation through to full commercial production. This ensures superior product quality and uniformity and creates a data rich environment across all sizes of freeze-drying systems. The next step forward in lyophilization, the science of freeze-drying, is being developed for pharmaceutical applications by use of enhanced technological capabilities, improved process and product understanding, and by implementing Line of Sight enabled freeze drying equipment.
Media Partners