Members Login

Channels
Special Offers & Promotions
Hemofarm Chooses Matrix Gemini Stability Management
Pharmaceutical company Hemofarm, based in Vršac, Serbia, has started a project with the Matrix Gemini Stability Management System from Autoscribe Informatics.
Hemofarm is a member of STADA Group, one of the largest generic pharmaceutical companies in the world. The Matrix Gemini Stability Management System will be for stability studies across Hemofarm’s rich product range of prescription drugs (Rx), over-the-counter medicines (OTC) and consumer healthcare products (CHC products).
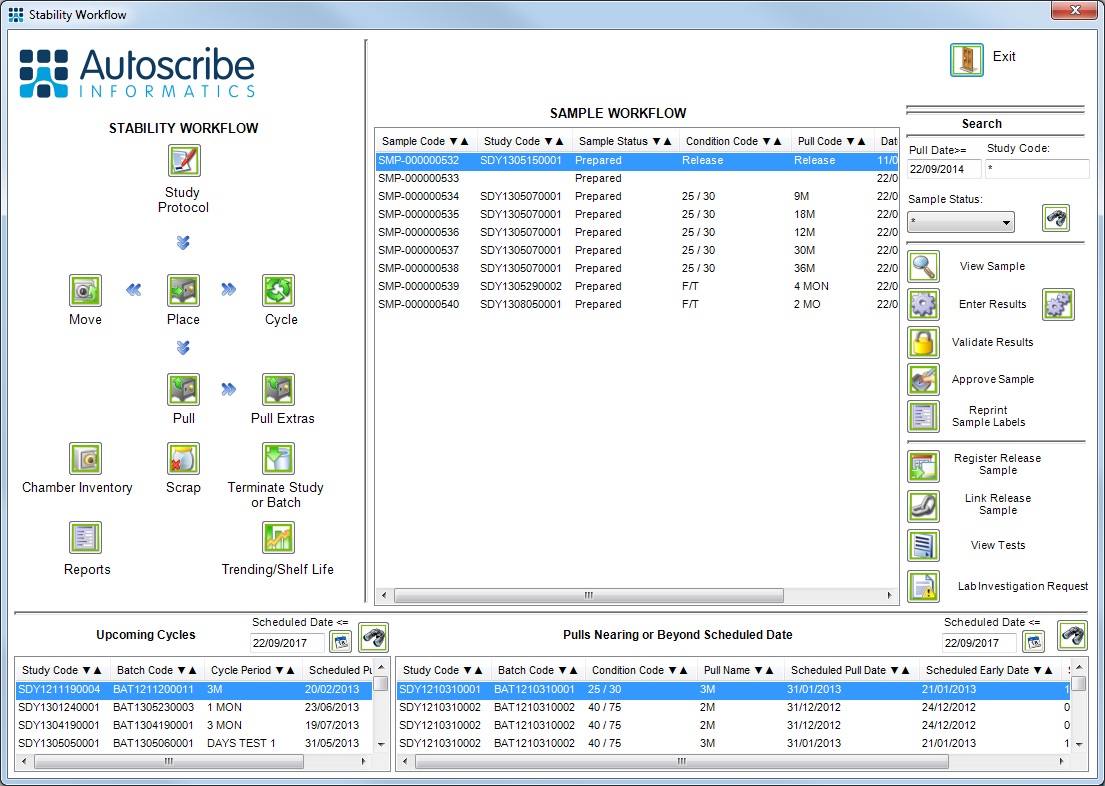
Based on the Matrix Gemini Laboratory Management System, Matrix Gemini Stability helps to simplify the whole stability study management process, which can be complex and time consuming for multiple studies. The in-built stability study management functionality is designed to automate and control the entire operation of the stability study including: protocol creation, study initiation and management, inventory management, sample pull points, future workload reporting and stability study reporting. The system can produce shelf life projections and is also optionally capable of producing projected shelf life trend analysis.
Founded in 1960, Hemofarm is one of the largest domestic producers and exporters of medicines in Serbia. It currently operates in 38 markets over 3 continents and has around 2,500 employees. Hemofarm became a part of STADA group in 2006 and has grown with STADA Group ever since, becoming an important member, especially in the Operations area.
John Boother, President of Autoscribe Informatics said, “We are pleased to be working with Hemofarm in their Stability Studies project. As one of the most complete and mature study management systems on the market today, Matrix Gemini Stability continues to gain traction and market share across the pharmaceutical industry. Its highly flexible graphical configuration tools ensure that the system can be configured to each customer’s exact needs, while providing the flexibility for change as the customer’s requirements evolve over time.”
Media Partners